Aldactazide® 25
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone During SARS-Cov-2 Infection
pharmaceuticals Review COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection Katarzyna Kotfis 1,* , Kacper Lechowicz 1 , Sylwester Drozd˙ zal˙ 2 , Paulina Nied´zwiedzka-Rystwej 3 , Tomasz K. Wojdacz 4, Ewelina Grywalska 5 , Jowita Biernawska 6, Magda Wi´sniewska 7 and Miłosz Parczewski 8 1 Department of Anesthesiology, Intensive Therapy and Acute Intoxications, Pomeranian Medical University in Szczecin, 70-111 Szczecin, Poland; [email protected] 2 Department of Pharmacokinetics and Monitored Therapy, Pomeranian Medical University, 70-111 Szczecin, Poland; [email protected] 3 Institute of Biology, University of Szczecin, 71-412 Szczecin, Poland; [email protected] 4 Independent Clinical Epigenetics Laboratory, Pomeranian Medical University, 71-252 Szczecin, Poland; [email protected] 5 Department of Clinical Immunology and Immunotherapy, Medical University of Lublin, 20-093 Lublin, Poland; [email protected] 6 Department of Anesthesiology and Intensive Therapy, Pomeranian Medical University in Szczecin, 71-252 Szczecin, Poland; [email protected] 7 Clinical Department of Nephrology, Transplantology and Internal Medicine, Pomeranian Medical University, 70-111 Szczecin, Poland; [email protected] 8 Department of Infectious, Tropical Diseases and Immune Deficiency, Pomeranian Medical University in Szczecin, 71-455 Szczecin, Poland; [email protected] * Correspondence: katarzyna.kotfi[email protected]; Tel.: +48-91-466-11-44 Abstract: In March 2020, coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was declared Citation: Kotfis, K.; Lechowicz, K.; a global pandemic by the World Health Organization (WHO). The clinical course of the disease is Drozd˙ zal,˙ S.; Nied´zwiedzka-Rystwej, unpredictable but may lead to severe acute respiratory infection (SARI) and pneumonia leading to P.; Wojdacz, T.K.; Grywalska, E.; acute respiratory distress syndrome (ARDS). -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -
Guidance for the Format and Content of the Protocol of Non-Interventional
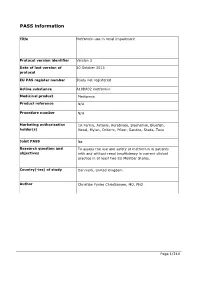
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Populationsweite Utilisationsuntersuchung in Den Chronischen Krankheitsbildern Hypertonie, Hyperlipid¨Amieund Typ 2 Diabetes Mellitus –
Ruhr-Universit¨atBochum Prof. Dr. rer. nat. Hans J. Trampisch Dienstort: Abteilung f¨urMedizinische Informatik, Biometrie und Epidemiologie Populationsweite Utilisationsuntersuchung in den chronischen Krankheitsbildern Hypertonie, Hyperlipid¨amieund Typ 2 Diabetes Mellitus { Eine Studie des Projekts PUKO-BHD an der Medizinischen Universit¨atWien Inaugural-Dissertation zur Erlangung des Doktorgrades der Medizin einer Hohen Medizinischen Fakult¨at der Ruhr-Universit¨atBochum vorgelegt von: Lisanne M. Jandeck aus Herford 2014 Dekan: Prof. Dr. med. Albrecht Bufe Referent: Prof. Dr. rer. nat. Hans J. Trampisch Korreferent: Prof. Dr. med. J¨urgenWindeler Tag der M¨undlichen Pr¨ufung:09.02.2017 Abstract Jandeck Lisanne M. Populationsweite Utilisationsuntersuchung in den chronischen Krankheitsbildern Hypertonie, Hyperlipid¨amieund Typ 2 Diabetes Mellitus Problem: Hypertonie (HT), Hyperlipid¨amie(HL) und Typ 2 Diabetes Mellitus (DM) stellen die h¨aufigstenchronischen Erkrankungen in Osterreich¨ dar, besonders bei Uber-50j¨ahrigen.Die¨ Zielsetzung dieser Teilstudie Utilisation\ des Projekts mit " dem Titel PUKO-BHD { Pr¨avalenz, Utilisation, Kosten und Outcome bei Blut- " hochdruck, Hyperlipid¨amieund Typ 2 Diabetes Mellitus\ ist eine populationsweite epidemiologische Untersuchung zu der Utilisation von Arzneimitteln zur Behand- lung dieser Krankheiten, insbesondere in Bezug auf Mehrfachverschreibungen (dou- ble prescriptions, DP) und Verschreibungen von Kombinationen von Wirkstoffen, die (schwerwiegende) Wechselwirkungen erzeugen k¨onnen (drug-drug -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Selection of a Mineralocorticoid Receptor Antagonist for Patients with Hypertension Or Heart Failure
European Journal of Heart Failure (2014) 16, 143–150 REVIEW doi:10.1111/ejhf.31 Selection of a mineralocorticoid receptor antagonist for patients with hypertension or heart failure Javaid Iqbal1*, Yasir Parviz1, Bertram Pitt2, John Newell-Price3, Abdallah Al-Mohammad1, and Faiez Zannad4 1Department of Cardiovascular Science at the University of Sheffield and Cardiology Department at Sheffield Teaching Hospitals NHS Trust, Sheffield,K; U 2Cardiovascular Centre, University of Michigan, Ann Arbor, MI, USA; 3Department of Human Metabolism at the University of Sheffield and Endocrinology Department at Sheffield Teaching Hospitals NHS Trust, Sheffield, UK; and 4INSERM, Centre d’Investigation Clinique and Centre Hospitalier Universitaire, and the Department of Cardiology, Nancy University, Université de Lorraine, Nancy, France Received 8 April 2013; revised 15July2013; accepted 19July2013; online publish-ahead-of-print 14 December 2013 Clinical trials have demonstrated morbidity and mortality benefits of mineralocorticoid receptor antagonists (MRAs) in patients with heart failure. These studies have used either spironolactone or eplerenone as the MRA. It is generally believed that these two agents have the same effects, and the data from studies using one drug could be extrapolated for the other. National and international guidelines do not generally discriminate between spironolactone and eplerenone, but strongly recommend using an MRA for patients with heart failure due to LV systolic dysfunction and post-infarct LV systolic dysfunction. There are no major clinical trials directly comparing the efficacy of these two drugs. This article aims to compare the pharmacokinetics and pharmacodynamics of spironolactone and eplerenone, and to analyse the available data for their cardiovascular indications and adverse effects. -

Aldactone® Spironolactone Tablets, USP
NDA 12-151/S-062 Page 2 Aldactone® spironolactone tablets, USP WARNING Aldactone has been shown to be a tumorigen in chronic toxicity studies in rats (see Precautions). Aldactone should be used only in those conditions described under Indications and Usage. Unnecessary use of this drug should be avoided. DESCRIPTION Aldactone oral tablets contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate, which has the following structural formula: Spironolactone is practically insoluble in water, soluble in alcohol, and freely soluble in benzene and in chloroform. Inactive ingredients include calcium sulfate, corn starch, flavor, hypromellose, iron oxide, magnesium stearate, polyethylene glycol, povidone, and titanium dioxide. ACTIONS / CLINICAL PHARMACOLOGY Mechanism of action: Aldactone (spironolactone) is a specific pharmacologic antagonist of aldosterone, acting primarily through competitive binding of receptors at the aldosterone-dependent sodium-potassium exchange site in the distal convoluted renal tubule. Aldactone causes increased amounts of sodium and water to be excreted, while potassium is retained. Aldactone acts both as a diuretic and as an antihypertensive drug by this mechanism. It may be given alone or with other diuretic agents which act more proximally in the renal tubule. Aldosterone antagonist activity: Increased levels of the mineralocorticoid, aldosterone, are present in primary and secondary hyperaldosteronism. Edematous states in which secondary aldosteronism is usually involved include congestive heart failure, hepatic cirrhosis, and the nephrotic syndrome. By competing with aldosterone for receptor sites, Aldactone provides effective therapy for the edema and ascites in those conditions. -

Progesterone and Related Progestins: Potential New Health Benefi Ts
CLIMACTERIC 2013;16(Suppl 1):69–78 Progesterone and related progestins: potential new health benefi ts R. Sitruk-Ware and M. El-Etr * Population Council and Rockefeller University, New York, NY, USA; * INSERM UMR 788, Kremlin Bic ê tre, France Key words: PROGESTERONE , PROGESTIN , NESTORONE, NEUROREGENERATION, MYELIN REPAIR, STROKE , HORMONE REPLACEMENT THERAPY, LACTATION , PROGESTERONE VAGINAL RING ABSTRACT Progesterone is a steroid hormone that is essential for the regulation of reproductive function. The main physiological roles of this hormone have been widely described. Progesterone and progestins have been approved for a number of indications including the treatment of irregular and anovulatory menstrual cycles and, when combined with estrogen, for contraception, and the prevention of endometrial hyperplasia in postmenopausal hormonal replacement therapy (HRT) regimens. Lack of understanding between the differ- ences in categories of the progestins as well as with the physiological hormone has resulted in considerable controversy surrounding the use of progestins for HRT regimens. Newer evidence suggests that there are distinct differences between the molecules and there is no progestin class effect, with regard to benefi ts or side-effects. In addition to its role in reproduction, progesterone regulates a number of biologically distinct processes in other tissues, particularly in the nervous system and the vessels. Recently, it has been shown in animal experiments that progesterone and the progestin Nestorone ® have positive effects on neuroregeneration and repair of brain damage, as well as myelin repair. The potential benefi ts of natural progesterone and its related derivatives warrant further investigation. It is hoped that a better understanding of the mechanism of action of progesterone and selected progestins will help in defi ning better therapies for men and women. -

SC-26304) (Kidney/Aldosterone Receptors/Tritiated Spirolactone) DIANA MARVER, JOHN STEWART*, JOHN W
Proc. Nat. Acad. Sci. USA Vol. 71, No. 4, pp. 1431-1435, April 1974 Renal Aldosterone Receptors: Studies with [3H]Aldosterone and the Anti-Mineralocorticoid [3H]Spirolactone (SC-26304) (kidney/aldosterone receptors/tritiated spirolactone) DIANA MARVER, JOHN STEWART*, JOHN W. FUNDERt, DAVID FELDMANT, AND ISIDORE S. EDELMAN Cardiovascular Research Institute and the Departments of Medicine, and of Biochemistry and Biophysics, University of California, San Francisco, Calif. 94143 Contributed by Isidre S. Edelman, January 30, 1974 ABSTRACT In vivo, a spirolactone (SC-26304) in- sterone binding site, to explore the characteristics of the aldo- hibited the effects of aldosterone on urinary K+: Na+ ratios sterone and and the binding of ['Hialdosterone to renal cytoplasmic receptor system the mechanism of the inhibitory and nuclear receptors. Cytoplasmic binding of ['flaldo_ effect (8). sterone and ['Hispirolactone (SC-26304) was similar in mag- nitude and involved the same set of sites. Under three MATERIALS AND METHODS sets of conditions-(i) in the intact rat, (ii) in kidney slices, and (iii) in reconstitution studies (mixing pre- Materials. 1,2-d-['H]Aldosterone (54 Ci/mmol) was ob- labeled cytoplasm with either purified renal nuclei or tained from New England Nuclear Corp., Boston, Mass. chromatin), ['Hispirolactone (SC-26304) did not yield [3H]SC-26304 (16 Ci/mmol) and unlabeled SC-26304 were specific nuclear complexes in contrast to the reproducible from the G. D. Searle generation of these complexes with [Hilaldosterone. In generous gifts Co., Chicago, Ill. Low glycerol density gradients, cytoplasmic ['Hialdosterone K+ food pellets (1.2 Aeq. of K+/g) were obtained from General receptor complexes sedimented at 8.5 S and 4 S in low Biochemical, Chagrin Falls, Ohio. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Drug-Induced Immune Hemolytic Anemia: the Last 30 Years of Changes P
George Garratty 1935–2014 Journal of Blood Group Serology and Molecular Genetics VOLUME 30, N UMBER 2, 2014 Immunohematology Journal of Blood Group Serology and Molecular Genetics Volume 30, Number 2, 2014 CONTENTS Introduction to Immunohematology Special Edition on 43 Drug-Induced Immune Cytopenias P.A. Arndt and R.M. Leger R EPORT 44 Drug-induced immune hemolytic anemia: the last 30 years of changes P. A . A r ndt R EPORT 55 Drug-induced immune thrombocytopenia: incidence, clinical features, laboratory testing, and pathogenic mechanisms B.R. Curtis R EPORT 66 Drugs that have been shown to cause drug-induced immune hemolytic anemia or positive direct antiglobulin tests: some interesting findings since 2007 G. Garratty and P.A. Arndt C A SE R EPORT 80 Diagnostic pitfalls of drug-induced immune hemolytic anemia A. Salama and B. Mayer R EPORT 85 How we investigate drug-induced immune hemolytic anemia R.M. Leger, P.A. Arndt, and G. Garratty R EPORT 95 Drug-induced immune neutropenia/agranulocytosis B.R. Curtis 102 A NNOUNCEMENTS 105 A DVERT I SEMENTS 109 I NSTRUCT I ONS FOR A UTHORS E D I TOR - I N -C H I EF E D I TOR ia L B OA RD Sandra Nance, MS, MT(ASCP)SBB Philadelphia, Pennsylvania Patricia Arndt, MT(ASCP)SBB Thierry Peyrard, PharmD, PhD Pomona, California Paris, France M A N AG I NG E D I TOR Barbara J. Bryant, MD Mark Popovsky, MD Cynthia Flickinger, MT(ASCP)SBB Milwaukee, Wisconsin Braintree, Massachusetts Philadelphia, Pennsylvania Lilian Castilho, PhD S. Gerald Sandler, MD Campinas, Brazil Washington, District of Columbia TECHN I C A L E D I TORS Christine Lomas-Francis, MSc Martha R.