Preliminary Vegetation Study of the Selous-Niassa Wildlife
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Museum of Economic Botany, Kew. Specimens Distributed 1901 - 1990
Museum of Economic Botany, Kew. Specimens distributed 1901 - 1990 Page 1 - https://biodiversitylibrary.org/page/57407494 15 July 1901 Dr T Johnson FLS, Science and Art Museum, Dublin Two cases containing the following:- Ackd 20.7.01 1. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 2. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 3. Wood of Melia indica, Anantapur, Paris Exhibition 1900 4. Wood of Anogeissus acuminata, Ganjam, Paris Exhibition 1900 5. Wood of Xylia dolabriformis, Godaveri, Paris Exhibition 1900 6. Wood of Pterocarpus Marsupium, Kistna, Paris Exhibition 1900 7. Wood of Lagerstremia parviflora, Godaveri, Paris Exhibition 1900 8. Wood of Anogeissus latifolia , Godaveri, Paris Exhibition 1900 9. Wood of Gyrocarpus jacquini, Kistna, Paris Exhibition 1900 10. Wood of Acrocarpus fraxinifolium, Nilgiris, Paris Exhibition 1900 11. Wood of Ulmus integrifolia, Nilgiris, Paris Exhibition 1900 12. Wood of Phyllanthus emblica, Assam, Paris Exhibition 1900 13. Wood of Adina cordifolia, Godaveri, Paris Exhibition 1900 14. Wood of Melia indica, Anantapur, Paris Exhibition 1900 15. Wood of Cedrela toona, Nilgiris, Paris Exhibition 1900 16. Wood of Premna bengalensis, Assam, Paris Exhibition 1900 17. Wood of Artocarpus chaplasha, Assam, Paris Exhibition 1900 18. Wood of Artocarpus integrifolia, Nilgiris, Paris Exhibition 1900 19. Wood of Ulmus wallichiana, N. India, Paris Exhibition 1900 20. Wood of Diospyros kurzii , India, Paris Exhibition 1900 21. Wood of Hardwickia binata, Kistna, Paris Exhibition 1900 22. Flowers of Heterotheca inuloides, Mexico, Paris Exhibition 1900 23. Leaves of Datura Stramonium, Paris Exhibition 1900 24. Plant of Mentha viridis, Paris Exhibition 1900 25. Plant of Monsonia ovata, S. -

First Molecular Phylogeny of the Pantropical Genus Dalbergia: Implications for Infrageneric Circumscription and Biogeography
SAJB-00970; No of Pages 7 South African Journal of Botany xxx (2013) xxx–xxx Contents lists available at SciVerse ScienceDirect South African Journal of Botany journal homepage: www.elsevier.com/locate/sajb First molecular phylogeny of the pantropical genus Dalbergia: implications for infrageneric circumscription and biogeography Mohammad Vatanparast a,⁎, Bente B. Klitgård b, Frits A.C.B. Adema c, R. Toby Pennington d, Tetsukazu Yahara e, Tadashi Kajita a a Department of Biology, Graduate School of Science, Chiba University, 1-33 Yayoi, Inage, Chiba, Japan b Herbarium, Library, Art and Archives, Royal Botanic Gardens, Kew, Richmond, United Kingdom c NHN Section, Netherlands Centre for Biodiversity Naturalis, Leiden University, Leiden, The Netherlands d Royal Botanic Garden Edinburgh, 20a Inverleith Row, Edinburgh, EH3 5LR, United Kingdom e Department of Biology, Kyushu University, Japan article info abstract Article history: The genus Dalbergia with c. 250 species has a pantropical distribution. In spite of the high economic and eco- Received 19 May 2013 logical value of the genus, it has not yet been the focus of a species level phylogenetic study. We utilized ITS Received in revised form 29 June 2013 nuclear sequence data and included 64 Dalbergia species representative of its entire geographic range to pro- Accepted 1 July 2013 vide a first phylogenetic framework of the genus to evaluate previous infrageneric classifications based on Available online xxxx morphological data. The phylogenetic analyses performed suggest that Dalbergia is monophyletic and that fi Edited by JS Boatwright it probably originated in the New World. Several clades corresponding to sections of these previous classi - cations are revealed. -

Habitats 0-Year
Table S1: The total number of recorded species per habitat (in brackets) and the number of shared species across habitats. 0-year- 3-year- 5-year- Restored Habitats Reference old old old combined 0-year-old (36 spp.) - 25 spp. 24 spp. - 25 spp. 3-year-old (40 spp.) 25 spp. - 28 spp. - 30 spp. 5-year-old (44 spp.) 24 spp. 28 spp. - - 40 spp. Restored combined (59 - - - - 49 spp. spp.) Reference (70 spp.) 25 spp. 30 spp. 40 spp. 49 spp. - Table S2: Checklist of plant species found in the restored and reference habitats. Species Family Upland area Lowland area 0-year- 3-year- 5-year- 0-year- 3-year- 5-year- old old old Reference old old old Reference habitat habitat habitat habiat habitat habitat habitat habitat Harpephyllum caffrum Bernh. Anacardiaceae X X X X X X X - Protorhus longifolia (Bernh.) Engl. Anacardiaceae - X X X - - - X Sclerocrya birrea (A.Rich.) Hochst. subsp. caffra (Sond.) Kokwaro Anacardiaceae - - - - - - - - Searsia chirindensis (Baker f.) Moffett Anacardiaceae - - X X - X X X Searsia dentata (Thunb.) F.A.Barkley Anacardiaceae - - - - - - - - Searsia lucida (L.) F.A.Barkley Anacardiaceae X - - - - X - - Searsia pentheri (Zahlbr.) Moffett Anacardiaceae - - X - - - - X Searsia rehmanniana (Engl.) Moffet Anacardiaceae X - - X - X X - Annona senegalensi Pers. Annonaceae - - - - - - - - Rauvolfia caffra Sond. Apocynaceae X X - X - - - X Tabernaemontana ventricosa Hochst. ex A.DC. Apocynaceae X X - X - - - X Cussonia spicata Thunb. Araliaceae - - - - - - - - Cussonia zuluensis Strey Araliaceae - - - X - - X - Phoenix reclinata Jacq. Arecaceae X X - - - - X X Aloe ferox Mill. Asphodelaceae - - X - - - X - Brachylaena discolor DC. Asteraceae X X X - - X - - Kigelia africana (Lam.) Benth. -

SSW Tree & Shrub Species List
SABI SAND WILDTUIN TREE & SHRUB SPECIES LIST Compiled by June Revised– December 2019 Candice2015 Pierce & Edwin Pierce Sabi Sand Wildtuin National Vernacular Name Scientific Notation Number 22 Wild Date Palm Hoenix reclinata 24 Northern Lala Palm Hyphaene petersiana 28.1 Krantz Aloe Aloe arborescens 29.5 Mountain Aloe Aloe marlothii 30.4 Lebombo Aloe Aloe spicata 37 Broad-leaved Waxberry Morella pilulifera 38 Lance-leaved Waxberry Morella serrata 39 White-stinkwood Celtis Africana 42 Pigeonwood Trema orientalis 43 Thorny-elm Chaetachme aristata 63 Large-leaved Rock Fig Ficus abutilifolia 48 Common Wild Fig Ficus burkei 64 Hairy Rock Fig Ficus glumosa 55 Red-leaved Fig Ficus ingens 48.1 Peters Fig Ficus petersii 60 Wonderboom Fig Ficus salicifolia 65 Lowveld Fig Ficus stuhlmannii National Vernacular Name Scientific Notation Number 50 Broom-cluster Fig Ficus sur 66 Sycomore Fig Ficus sycomorus 70 Rock Tree Nettle Obetia tenax 71 Soap Nettle Pouzolzia mixta 73 Escarpment Beechwood Faurea galpinii 76 Broad-leaved Beechwood Faurea rochetiana 75 Willow Beechwood Faurea saligna 100 Rock Tannin-bush Osyris lanceolata 101 Small-fruit Olax Olax dissitiflora 102 Blue Sourplum Ximenia americana 103 Sourplum Ximenia caffra 104 Spekboom Portulacaria afra 106 Shakama-plum Hexalobus monopetalus 105 Wild Custard-apple Annona senegalensis 130 Hedge Caper-bush Capparis sepiaria 130.1 Woolly Caper-bush Capparis tomentosa 122 Shepherds-tree Boscia albitrunca 129.1 Green-leaved Wormbush Cadaba natalensis 129.3 Grey-leaved Wormbush Cadaba termitaria 132 Bead-bean -

Albany Thicket Biome
% S % 19 (2006) Albany Thicket Biome 10 David B. Hoare, Ladislav Mucina, Michael C. Rutherford, Jan H.J. Vlok, Doug I.W. Euston-Brown, Anthony R. Palmer, Leslie W. Powrie, Richard G. Lechmere-Oertel, Şerban M. Procheş, Anthony P. Dold and Robert A. Ward Table of Contents 1 Introduction: Delimitation and Global Perspective 542 2 Major Vegetation Patterns 544 3 Ecology: Climate, Geology, Soils and Natural Processes 544 3.1 Climate 544 3.2 Geology and Soils 545 3.3 Natural Processes 546 4 Origins and Biogeography 547 4.1 Origins of the Albany Thicket Biome 547 4.2 Biogeography 548 5 Land Use History 548 6 Current Status, Threats and Actions 549 7 Further Research 550 8 Descriptions of Vegetation Units 550 9 Credits 565 10 References 565 List of Vegetation Units AT 1 Southern Cape Valley Thicket 550 AT 2 Gamka Thicket 551 AT 3 Groot Thicket 552 AT 4 Gamtoos Thicket 553 AT 5 Sundays Noorsveld 555 AT 6 Sundays Thicket 556 AT 7 Coega Bontveld 557 AT 8 Kowie Thicket 558 AT 9 Albany Coastal Belt 559 AT 10 Great Fish Noorsveld 560 AT 11 Great Fish Thicket 561 AT 12 Buffels Thicket 562 AT 13 Eastern Cape Escarpment Thicket 563 AT 14 Camdebo Escarpment Thicket 563 Figure 10.1 AT 8 Kowie Thicket: Kowie River meandering in the Waters Meeting Nature Reserve near Bathurst (Eastern Cape), surrounded by dense thickets dominated by succulent Euphorbia trees (on steep slopes and subkrantz positions) and by dry-forest habitats housing patches of FOz 6 Southern Coastal Forest lower down close to the river. -

Emironmera: Thnntlofjb1l~1NI
THE RECOVERY OF AN AFROMONTANE FOREST AFTER SLASH AND BURN: A.CASE STUDY FROM THE NKANDLA FOREST CIEI1'\ITRE FOR emironmera: tHnntLOfJb1l~1NI by WADE MACPHERSON Submitted in partial fulfilment ofthe academic requirements ofthe degree ofMasters of Science in the Centre for Environment and Development University ofNatal, Pietermaritzburg December 2000 This project was carried out within the Forest Biodiversity Programme School of Botany and Zoology University of Natal, Pietermaritzburg BiODiVERSiTY PROGRAMME · 111 Preface The research presented in this dissertation was conducted in the Forest Biodiversity Programme as part of the requirements for the degree ofMaster of Science in the Centre for Environment and Development, University of Natal, Pietermaritzburg. The research was carried out from August 1999 to December 2000, under the eo-supervision of Prof M. J. Lawes, Dr J. E. Granger and Dr T. Hill. These studies represent the original work by the author and have not otherwise been submitted in any other form for any degree or diploma to another university. Where use has been made ofthe work ofothers, it is duly acknowledged in the text. Signed Wade Macpherson IV Abstract Clear cutting and subsequent agricultural processes lead not only to species loss, but also have negative consequences for soil and water conservation. This study investigated the response to, and effect of, deforestation due to shifting cultivation on the Nkandla forest complex (NFC), a subtropical Afromontane forest. AB a consequence of shifting agriculture, abandoned areas are nutrient poor, have depleted seed banks and may lack the ability to regenerate into species rich mature forest. The ability of forests to regenerate after disturbance effects is extremely important for the maintenance of ecological diversity and the stability of forest ecosystems. -

Dalbergia Species Currently Held in Species + and a Full List of Source References in Response to a Request by the Nomenclature Specialist
Original language: English PC24 Inf. 15 (English only/Seulement en anglais/Únicamente en inglés) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ___________________ Twenty-fourth meeting of the Plants Committee Geneva (Switzerland), 20, 21 and 23-26 July 2018 Species specific matters Maintenance of the Appendices PLANT NOMENCLATURE This information document has been submitted by the Secretariat on behalf of the United Nations Environment Programme-World Conservation Monitoring Centre (UNEP-WCMC) in relation to agenda item 27.* * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. PC24 Inf. 15 – p. 1 This information document provides information on plant nomenclature, specifically in relation to: 1. A response by UNEP-WCMC to CoP17 Decision 17.315, directed to the Secretariat, requesting feedback on the CITES Cactaceae Checklist (3rd edition) adopted at CoP17 from UNEP-WCMC. 2. An output of names of the Dalbergia species currently held in Species + and a full list of source references in response to a request by the Nomenclature Specialist. 3. An overview of data management approaches used for CITES-related data within Species+ (such as treatment of synonyms, hybrids etc.). This addresses recent requests by Parties on management of data for plants within Species+, as well as an action outlined in PC23 Com. -
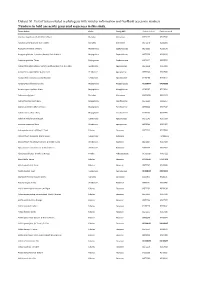
Dataset S1. List of Taxa Included in Phylogeny with Voucher Information and Genbank Accession Numbers
Dataset S1. List of taxa included in phylogeny with voucher information and GenBank accession numbers. Numbers in bold are newly generated sequences in this study. Taxon Author Order Family APG Genbank rbcLa Genbank matK Abutilon angulatum (Guill. & Perr.) Mast. Malvales Malvaceae JX572177 JX517944 Abutilon sonneratianum (Cav.) Sweet Malvales Malvaceae JX572178 JX518201 Acalypha chirindica S.Moore Malpighiales Euphorbiaceae JX572236 JX518178 Acalypha glabrata f. pilosior (Kuntze) Prain & Hutch. Malpighiales Euphorbiaceae JX572238 JX518120 Acalypha glabrata Thunb. Malpighiales Euphorbiaceae JX572237 JX517655 Acokanthera oblongifolia (Hochst.) Benth. & Hook.f. ex B.D.Jacks. Gentianales Apocynaceae JX572239 JX517911 Acokanthera oppositifolia (Lam.) Codd Gentianales Apocynaceae JX572240 JX517680 Acokanthera rotundata (Codd) Kupicha Gentianales Apocynaceae JF265266 JF270623 Acridocarpus chloropterus Oliv. Malpighiales Malpighiaceae KU568077 KX146299 Acridocarpus natalitius A.Juss. Malpighiales Malpighiaceae JF265267 JF270624 Adansonia digitata L. Malvales Malvaceae JQ025018 JQ024933 Adenia fruticosa Burtt Davy Malpighiales Passifloraceae JX572241 JX905957 Adenia gummifera (Harv.) Harms Malpighiales Passifloraceae JX572242 JX517347 Adenia spinosa Burtt Davy Malpighiales Passifloraceae JF265269 JX905950 Adenium multiflorum Klotzsch Gentianales Apocynaceae JX572243 JX517509 Adenium swazicum Stapf Gentianales Apocynaceae JX572244 JX517457 Adenopodia spicata (E.Mey.) C.Presl Fabales Fabaceae JX572245 JX517808 Afrocanthium lactescens (Hiern) Lantz -

Academica Supplementum 2 2012 Issn 0587-2405
ACTA ACADEMICA SUPPLEMENTUM 2 2012 ISSN 0587-2405 ACADEMICA SUPPLEMENTUM 2012 2 ACTA ACADEMICA SUPPLEMENTUM 2012: 2 Bushman (San) influence on Zulu place names Peter E. Raper DIE UNIVERSITEIT VAN DIE VRYSTAAT THE UNIVERSITY OF THE FREE STATE Instructions to authors Redaksie/Editorial Staff 1. Acta Academica publishes articles in Van Rensburg 1986: 1-34. Redakteur/Editor D J van den Berg (UV/UFS) Afrikaans or English. The preferred length is Van Rensburg C D (ed) Assistentredakteurs/ H Alt J K Coetzee N C de Wet J S du Toit about 7 000 words; 4 500 words is regarded as 1986. Human rights in South Africa. 2nd Assistant Editors J P Fouché M Kriel N Morgan P Duvehage a minimum and 11 000 as a maximum. ed. Pretoria: HAUM. J P C van den Berg M Viljoen G E Visser (UV/UFS) 2. Two printouts of the text as well as a file 9. Abbreviations and acronyms should on computer disc in MS-Word for Windows be avoided (except where an acronym, e.g. should be submitted. Articles may also be SABC, is current parlance). submitted by e-mail to [email protected]. 10. Italics should not be over-used for Redaksionele Medewerkers/Editorial Associates 3. Articles are to be submitted ready for emphasis. Latin phrases such as per se must J Baetens (Katholieke U, Leuven) D K Behera (Sambalpur U, India) the press: finally edited, stylistically polished be italicised. Words in languages other than S J Berkhout (US) and carefully proofread. Readability, fluency that of the manuscript should be given in A L Combrink (UNW: Potchkampus/Potch Campus) of style and clarity of exposition are essential. -
Plant Studies
PPLLAANNTT SSTTUUDDIIEESS IINNTTRROODDUUCCTTIIOONN TTOO TTRREEEESS AANNDD GGRRAASSSSEESS SSEECCTTIIOONN 22 IInnttrroodduuccttiioonn ttoo TTrreeeess –– PPaarrtt 11 All rights reserved. No part of this material may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and recording by any informational retrieval system, without the permission of the author. BUSHVELD TRAINING ADVENTURES © 2006/7/8 BUSHVELD TRAINING ADVENTURES INTRODUCTION TO TREES AND GRASSES 2 TABLE OF CONTENTS 1. OUTCOMES FOR THIS MODULE...................................................................................3 2. NOTES..........................................................................................................................3 3. INTRODUCTION TO TREES..........................................................................................4 4. WHAT IS A TREE ? .......................................................................................................4 5. TREE IDENTIFICATION ...............................................................................................4 6. LEAVES ........................................................................................................................5 6.1. Simple Leaves ............................................................................................................5 6.2. Compound Leaves ......................................................................................................5 6.2.1. Pinnately compound leaves ............................................................................5 -
Restoration of a Degraded Subtropical Forest for Climate Change Mitigation and Adaptation in the City of Durban, South Africa
RESTORATION OF A DEGRADED SUBTROPICAL FOREST FOR CLIMATE CHANGE MITIGATION AND ADAPTATION IN THE CITY OF DURBAN, SOUTH AFRICA Lutendo F. Mugwedi Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in Environmental Science (Specialization: Forest restoration ecology) School of Agricultural, Earth and Environmental Sciences College of Agriculture, Engineering and Science University of KwaZulu-Natal Pietermaritzburg Campus South Africa December 2017 PREFACE The research contained in this thesis was completed by the candidate while based in the Discipline of Land Use Planning and Management, School of Agricultural, Earth and Environmental Sciences, in the college of Agriculture, Engineering and Science, University of KwaZulu-Natal, Pietermaritzburg Campus, South Africa. The research was supported by eThekwini Municipality through the Durban Research Action Partnership: Community Reforestation Research Programme, and by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa. The contents of this work have not been submitted in any form to another university and, except where the work of others is acknowledged in the text, the results reported are due to investigations by the candidate. Lutendo F. Mugwedi Signed: Date: 04 December 2017 Supervisor: Prof. M. Rouget Signed: Date: 04 December 2017 Co-supervisors: Dr. B. Egoh Signed: Date: 04 December 2017 Prof. R. Slotow Signed: Date: 04 December 2017 Dr. S. Naidoo Signed: Date: -

Dalbergia Proposal Guatemala
CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II A. Proposal Inclusion of the genus Dalbergia in CITES Appendix II with exception to the species included in Appendix I. The UNEP-WCMC assessed the Dalbergia species of Latin America and concluded: “… all populations of Dalbergia spp. from South and Central America appear to meet the criteria for listing in CITES Appendix II” (UNEP-WCMC, 2015). Including the whole genus in Appendix II will be essential for the control of international trade by eliminating the arduous task of enforcement and customs officers of differentiating between the hundreds of Dalbergia species listed and not listed in CITES. The inclusion will help ensure that legal trade does not become a direct cause of the extinction of these highly threatened species and will help curb illegal trade. Considering that CITES Appendix II must include all species, which although not necessarily now threatened with extinction may become so unless trade in specimens of such species is subject to strict regulation in order to avoid utilization incompatible with their survival, it is important to include the genus Dalbergia in CITES Appendix II. a) Resolution Conf. 9.24, Annex 2 a, Criterion A - ”It is known, or can be inferred or projected, that the regulation of trade in the species is necessary to avoid it becoming eligible for inclusion in Appendix I in the near future”. b) Resolution Conf. 9.24, Annex 2 a, Criterion B - ”It is known, or can be inferred or projected, that regulation of trade in the species is required to ensure that the harvest of specimens from the wild is not reducing the wild population to a level at which its survival might be threatened by continued harvesting or other influences”.