FOI Summary, Thianil (Thiafentanil Oxalate), MIF 900-000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
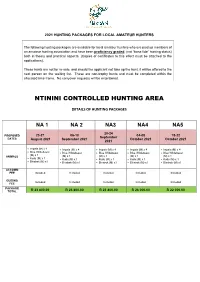
Ntinini Controlled Hunting Area
2021 HUNTING PACKAGES FOR LOCAL AMATEUR HUNTERS The following hunting packages are available for local amateur hunters who are paid up members of an amateur hunting association and have been proficiency graded, (not “bona fide” hunting status) both in theory and practical aspects. (Copies of certificates to this effect must be attached to the applications). These hunts are not for re-sale, and should the applicant not take up the hunt, it will be offered to the next person on the waiting list. These are non-trophy hunts and must be completed within the allocated time frame. No carryover requests will be entertained. NTININI CONTROLLED HUNTING AREA DETAILS OF HUNTING PACKAGES NA 1 NA 2 NA3 NA4 NA5 20-24 23-27 06-10 04-08 18-22 PROPOSED September DATES August 2021 September 2021 October 2021 October 2021 2021 • Impala (M) x 4 • Impala (M) x 4 • Impala (M) x 4 • Impala (M) x 4 • Impala (M) x 4 • Blue Wildebeest • Blue Wildebeest • Blue Wildebeest • Blue Wildebeest • Blue Wildebeest (M) x 1 ANIMALS (M) x 1 (M) x 1 (M) x 1 (M) x 1 • Kudu (M) x 1 • Kudu (M) x 1 • Kudu (M) x 1 • Kudu (M) x 1 • Kudu (M) x 1 • Blesbok (M) x1 • Blesbok (M) x1 • Blesbok (M) x1 • Blesbok (M) x1 • Blesbok (M) x1 ACCOMM FEE Included Included Included Included Included GUIDING Included Included Included Included Included FEE PACKAGE TOTAL R 23 800.00 R 23 800.00 R 23 800.00 R 26 000.00 R 22 000.00 ITHALA CONTROLLED HUNTING AREA DETAILS OF HUNTING PACKAGES IGRA 1 IGRA 2 IGRA 3 IGRA 4 PROPOSED 11-16 02-07 16-21 06-11 DATES June 2021 July 2021 July 2021 August 2021 • Impala -

Foot and Mouth Disease Fastfact
Foot and Mouth Disease (FMD) What is foot and mouth How does FMD affect my Who should I contact if I disease and what causes it? animal? suspect FMD? Foot and mouth disease (FMD) is The most common signs of foot Contact your veterinarian a highly contagious viral disease of and mouth disease are fever and immediately. FMD is not currently cloven-hoofed (two-toed) animals (e.g., the formation of blisters, ulcers and found in the United States; suspicion cattle, pigs, sheep). FMD causes painful sores on the mouth, tongue, nose, of the disease requires immediate sores and blisters on the feet, mouth feet, and teats. Foot lesions occur in attention. the area of the coronary band and and teats of animals. between the toes. Infected cattle are How can I protect my animal Foot and mouth disease is a high depressed, reluctant to move, and from FMD? consequence livestock disease due to unwilling or unable to eat, which can To prevent the introduction of FMD, its potential for rapid spread, severe lead to decreased milk production, use strict biosecurity procedures on trade restrictions and the subsequent weight loss, and poor growth. Affected your farm. Isolate any new introductions economic impacts that would result. animals may also have nasal discharge or animal returning to the farm for The disease occurs in parts of Asia, and excessive salivation. Pigs often several weeks before introducing them have sore feet but less commonly into the herd. Minimize visitors on Africa, the Middle East and South develop mouth lesions. Sheep and your farm, especially those that have America, but has been eradicated goats show very mild, if any, signs of traveled from countries with FMD. -

Pending World Record Waterbuck Wins Top Honor SC Life Member Susan Stout Has in THIS ISSUE Dbeen Awarded the President’S Cup Letter from the President
DSC NEWSLETTER VOLUME 32,Camp ISSUE 5 TalkJUNE 2019 Pending World Record Waterbuck Wins Top Honor SC Life Member Susan Stout has IN THIS ISSUE Dbeen awarded the President’s Cup Letter from the President .....................1 for her pending world record East African DSC Foundation .....................................2 Defassa Waterbuck. Awards Night Results ...........................4 DSC’s April Monthly Meeting brings Industry News ........................................8 members together to celebrate the annual Chapter News .........................................9 Trophy and Photo Award presentation. Capstick Award ....................................10 This year, there were over 150 entries for Dove Hunt ..............................................12 the Trophy Awards, spanning 22 countries Obituary ..................................................14 and almost 100 different species. Membership Drive ...............................14 As photos of all the entries played Kid Fish ....................................................16 during cocktail hour, the room was Wine Pairing Dinner ............................16 abuzz with stories of all the incredible Traveler’s Advisory ..............................17 adventures experienced – ibex in Spain, Hotel Block for Heritage ....................19 scenic helicopter rides over the Northwest Big Bore Shoot .....................................20 Territories, puku in Zambia. CIC International Conference ..........22 In determining the winners, the judges DSC Publications Update -

Population, Distribution and Conservation Status of Sitatunga (Tragelaphus Spekei) (Sclater) in Selected Wetlands in Uganda
POPULATION, DISTRIBUTION AND CONSERVATION STATUS OF SITATUNGA (TRAGELAPHUS SPEKEI) (SCLATER) IN SELECTED WETLANDS IN UGANDA Biological -Life history Biological -Ecologicl… Protection -Regulation of… 5 Biological -Dispersal Protection -Effectiveness… 4 Biological -Human tolerance Protection -proportion… 3 Status -National Distribtuion Incentive - habitat… 2 Status -National Abundance Incentive - species… 1 Status -National… Incentive - Effect of harvest 0 Status -National… Monitoring - confidence in… Status -National Major… Monitoring - methods used… Harvest Management -… Control -Confidence in… Harvest Management -… Control - Open access… Harvest Management -… Control of Harvest-in… Harvest Management -Aim… Control of Harvest-in… Harvest Management -… Control of Harvest-in… Tragelaphus spekii (sitatunga) NonSubmitted Detrimental to Findings (NDF) Research and Monitoring Unit Uganda Wildlife Authority (UWA) Plot 7 Kira Road Kamwokya, P.O. Box 3530 Kampala Uganda Email/Web - [email protected]/ www.ugandawildlife.org Prepared By Dr. Edward Andama (PhD) Lead consultant Busitema University, P. O. Box 236, Tororo Uganda Telephone: 0772464279 or 0704281806 E-mail: [email protected] [email protected], [email protected] Final Report i January 2019 Contents ACRONYMS, ABBREVIATIONS, AND GLOSSARY .......................................................... vii EXECUTIVE SUMMARY ....................................................................................................... viii 1.1Background ........................................................................................................................... -

Influence of Common Eland (Taurotragus Oryx) Meat Composition on Its Further Technological Processing
CZECH UNIVERSITY OF LIFE SCIENCES PRAGUE Faculty of Tropical AgriSciences Department of Animal Science and Food Processing Influence of Common Eland (Taurotragus oryx) Meat Composition on its further Technological Processing DISSERTATION THESIS Prague 2018 Author: Supervisor: Ing. et Ing. Petr Kolbábek prof. MVDr. Daniela Lukešová, CSc. Co-supervisors: Ing. Radim Kotrba, Ph.D. Ing. Ludmila Prokůpková, Ph.D. Declaration I hereby declare that I have done this thesis entitled “Influence of Common Eland (Taurotragus oryx) Meat Composition on its further Technological Processing” independently, all texts in this thesis are original, and all the sources have been quoted and acknowledged by means of complete references and according to Citation rules of the FTA. In Prague 5th October 2018 ………..………………… Acknowledgements I would like to express my deep gratitude to prof. MVDr. Daniela Lukešová CSc., Ing. Radim Kotrba, Ph.D. and Ing. Ludmila Prokůpková, Ph.D., and doc. Ing. Lenka Kouřimská, Ph.D., my research supervisors, for their patient guidance, enthusiastic encouragement and useful critiques of this research work. I am very gratefull to Ing. Petra Maxová and Ing. Eva Kůtová for their valuable help during the research. I am also gratefull to Mr. Petr Beluš, who works as a keeper of elands in Lány, Mrs. Blanka Dvořáková, technician in the laboratory of meat science. My deep acknowledgement belongs to Ing. Radek Stibor and Mr. Josef Hora, skilled butchers from the slaughterhouse in Prague – Uhříněves and to JUDr. Pavel Jirkovský, expert marksman, who shot the animals. I am very gratefull to the experts from the Natura Food Additives, joint-stock company and from the Alimpex-maso, Inc. -

The Opioid Epidemic: What Labs Have to Do with It?
The Opioid Epidemic: What labs have to do with it? Ewa King, Ph.D. Associate Director of Health RIDOH State Health Laboratories Analysis. Answers. Action. www.aphl.org Overview • Overdose trends • Opioids and their effects • Analytical testing approaches • Toxicology laboratories Analysis. Answers. Action. www.aphl.org Opioid overdose crisis 1 Analysis. Answers. Action. www.aphl.org Opioid overdose crisis 2 Analysis. Answers. Action. www.aphl.org Opiates and Opioids • Opiates vs. Opioids • Opiates: Naturally occurring, derived from the poppy plant • Opioids: “Opiate-like” drugs in effects, not chemical structure Includes opiates • Narcotic analgesics • CNS depressants • DEA Schedule I or II controlled substances • Additive effect with other CNS depressant drugs Analysis. Answers. Action. www.aphl.org Efficacy of Opioids • How do opioids work? • Bind with opioid receptors • Brain, spinal cord, GI tract, and throughout the body • Pain, emotion, breathing, movement, and digestion Opioid Receptor Analysis. Answers. Action. www.aphl.org Effects of Opioids Physiological Psychological • Pain relief • Drowsiness/ sedation • Cough suppression • Mental confusion • GI motility • Loss of memory • Respiratory depression • Lethargy/ apathy • Pupillary constriction • Euphoria/ tranquility • Itching • Mood swings • Constipation • Depression • Dependence • Withdrawal • Dependence Analysis. Answers. Action. www.aphl.org Opiates 1 Opiates • Naturally occurring alkaloids Opium • Latex from the opium poppy plant Codeine: • Mild to moderate pain • Antitussive Morphine: • Severe pain • Metabolite of codeine and heroin Analysis. Answers. Action. www.aphl.org Opiates 2 Semi-synthetic Opiates: • Synthesized from a natural opiate Heroin: • Schedule I narcotic Hydrocodone (Vicodin): • Mild to moderate pain • Metabolizes to hydromorphone (Dilaudid) Oxycodone (Oxycontin/Percocet): • Moderate to severe pain • Metabolizes to oxymorphone (Opana) Analysis. Answers. Action. -

A Review of Unique Opioids and Their Conversions
A Review of Unique Opioids and Their Conversions Jacqueline Cleary, PharmD, BCACP Assistant Professor Albany College of Pharmacy and Health Sciences Adjunct Professor SAGE College of Nursing DISCLOSURES • Kaleo • Remitigate, LLC OBJECTIVES • Compare and contrast unique pharmacotherapy options for the treatment of chronic pain including: methadone, buprenoprhine, tapentadol, and tramadol • Select methadone, buprenorphine, tapentadol, or tramadol based on patient specific factors • Apply appropriate opioid conversion strategies to unique opioids • Understand opioid overdose risk surrounding opioid conversions and the use of unique opioids UNIQUE OPIOIDS METHADONE, BUPRENORPHINE, TRAMADOL, TAPENTADOL METHADONE My favorite drug because….? METHADONE- INDICATIONS • FDA labeled indications – (1) chronic pain (2) detoxification Oral soluble tablets for suspension NOT indicated for chronic pain treatment • Initial inpatient detoxification of opioids by a licensed trained provider with methadone and supportive care is appropriate • Methadone maintenance provider must have special credentialing and training as required by state Outpatient prescription must be for pain ONLY and say “for pain” on RX • Continuation of methadone maintenance from outside provider while patient is inpatient for another condition is appropriate http://cdn.atforum.com/wp-content/uploads/SAMHSA-2015-Guidelines-for-OTPs.pdf MECHANISM OF ACTION • Potent µ-opioid agonist • NMDA receptor antagonist • Norepinephrine reuptake inhibitor • Serotonin reuptake inhibitor ADVERSE EVENTS -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Opioid & Other Drug Overdose Syndromic Surveillance Report
Opioid & Other Drug Overdose Syndromic Surveillance Report Suspected Unintentional Overdose Visits Past 14 Weeks Week Starting # of Visits 7-Week Ave. Alerts (1-Week Lag) 6/20/21 32 33.0 None 6/27/21 39 33.4 None 7/4/21 27 36.0 None 7/11/21 35 35.1 None 7/18/21 31 35.9 None 7/25/21 38 34.6 None 8/1/21 26 33.7 None 8/8/21 37 32.6 None 8/15/21 30 33.3 None 8/22/21 31 32.0 None 8/29/21 24 32.6 None 9/5/21 33 31.0 None 9/12/21 22 31.3 None 9/19/21 26 29.0 None Date report produced: 27-Sep-2021 Notes. Includes all patients 10 years and older visiting ALL Simcoe Muskoka Hospitals. Opioid and/or Toxicity related visits with a CTAS score of 1, 2, 3 or missing. Alerts are generated using the CDC EARS CUSUM Algorithm. C1 uses a 7-Week baseline with a one-Week lag. Participating Hospitals. Muskoka Algonquin Healthcare – Bracebridge (BRSH) & Muskoka Algonquin Healthcare – Huntsville (HUSH); Georgian Bay General Hospital (GBGH) & Orillia Soldiers Memorial Hospital (OSMH); Royal Victoria Regional Health Centre (RVH); Stevenson Memorial Hospital (SVMH); Collingwood General and Marine Hospital (CGMH). Data Source. Acute Care Enhanced Surveillance (ACES): a real-time syndromic surveillance system developed and maintained by Kingston, Frontenac and Lennox and Addington (KFL&A) Public Health and funded by the Ministry of Health and Long Term Care (http://www.kflaphi.ca/acute-care-enhanced-surveillance/). -

For Review Only 440 IUCN SSC Antelope Specialist Group
Manuscripts submitted to Journal of Mammalogy A Pliocene hybridisation event reconciles incongruent mitochondrial and nuclear gene trees among spiral-horned antelopes Journal:For Journal Review of Mammalogy Only Manuscript ID Draft Manuscript Type: Feature Article Date Submitted by the Author: n/a Complete List of Authors: Rakotoarivelo, Andrinajoro; University of Venda, Department of Zoology O'Donoghue, Paul; Specialist Wildlife Services, Specialist Wildlife Services Bruford, Michael; Cardiff University, Cardiff School of Biosciences Moodley, Yoshan; University of Venda, Department of Zoology adaptive radiation, interspecific hybridisation, paleoclimate, Sub-Saharan Keywords: Africa, Tragelaphus https://mc.manuscriptcentral.com/jmamm Page 1 of 34 Manuscripts submitted to Journal of Mammalogy 1 Yoshan Moodley, Department of Zoology, University of Venda, 2 [email protected] 3 Interspecific hybridization in Tragelaphus 4 A Pliocene hybridisation event reconciles incongruent mitochondrial and nuclear gene 5 trees among spiral-horned antelopes 6 ANDRINAJORO R. RAKATOARIVELO, PAUL O’DONOGHUE, MICHAEL W. BRUFORD, AND * 7 YOSHAN MOODLEY 8 Department of Zoology, University of Venda, University Road, Thohoyandou 0950, Republic 9 of South Africa (ARR, ForYM) Review Only 10 Specialist Wildlife Services, 102 Bowen Court, St Asaph, LL17 0JE, United Kingdom (PO) 11 Cardiff School of Biosciences, Sir Martin Evans Building, Cardiff University, Museum 12 Avenue, Cardiff, CF10 3AX, United Kingdom (MWB) 13 Natiora Ahy Madagasikara, Lot IIU57K Bis, Ampahibe, Antananarivo 101, Madagascar 14 (ARR) 15 16 1 https://mc.manuscriptcentral.com/jmamm Manuscripts submitted to Journal of Mammalogy Page 2 of 34 17 ABSTRACT 18 The spiral-horned antelopes (Genus Tragelaphus) are among the most phenotypically diverse 19 of all large mammals, and evolved in Africa during an adaptive radiation that began in the late 20 Miocene, around 6 million years ago. -

Rangifer Tarandus) Hoof Suitable for Efficient Locomotion on Complex Grounds
J Vet Res 61, 223-229, 2017 DE DE GRUYTER OPEN DOI:10.1515/jvetres-2017-0029 G Macro-microscopic research in reideer (Rangifer tarandus) hoof suitable for efficient locomotion on complex grounds Rui Zhang, Yu Qiao, Qiaoli Ji, Songsong Ma, Jianqiao Li Key Laboratory of Bionic Engineering, Ministry of Education, Jilin University, Nanguan District, Changchun, 130022, People's Republic of China [email protected] Received: November 25, 2016 Accepted: May 8, 2017 Abstract Introduction: Reindeer are adapted to long distance migration. This species can cope with variations in substrate, especially in ice and snow environment. However, few detailed studies about reindeer hoof are available. Thus this article describes the results of studies on macro- and micro-structures of reindeer hoof. Material and Methods: The gross anatomy of the reindeer hooves was examined. Stereo microscope (SM) and a scanning electron microscope (SEM) were used to observe four key selected positions of reindeer hooves. Moreover, element contents of the three selected positions of reindeer hooves were analysed using the SEM equipped with energy dispersive spectroscope. Results: Hoof bone structures were similar to other artiodactyl animals. In the microscopic analysis, the surfaces of the ungula sphere and ungula sole presented irregular laminated structure. Ungula edge surfaces were smooth and ungula cusp surfaces had unique features. Aside from C, O, and N, reindeer hooves contained such elements as S, Si, Fe, Al, and Ca. The content of the elements in different parts varied. Ti was the particular element in the ungula sole, and ungula edge lacked Mg and S which other parts contained. -

CAMEROON Savanna & Forest
Serving Sportsmen Since 1952 CAMEROON Savanna & Forest Hunting safaris in Cameroon are conducted in both the Savannah region which is generally in the North of the country and the Forest in the Southern regions. The areas are safe and easily accessible. Cameroon is an incredible country for big game hunting and we arrange safaris in the best hunting areas of the country. Safari Outfitters has represented this superb hunting outfitter Corporate Headquarters longer then any of our other African outfitters with 143 South Bent Street, Suite D the exception of one. So you will see that we know Powell, WY 82435 them very well and are 100% confident in their 307-587-5596 areas, success, PH’s and camps. www.safari1.com cf Breaking Records... it’s what we do! Savannah Forest Without a doubt, the magnificent Lord Derby or Arguably the most strikingly beautiful antelope in Giant Eland is one the top trophies in all of Africa all of Africa, the Bongo is high on the list of those and the largest of the antelope group. This guy can who yearn to hunt wild places. Local Pygmies trot all day and jump a 6 foot fence from a standing trackers and their small dogs are used to hunt start. In addition to Lord Derby Eland, game such Bongo in the dense rainforest and they do an as Western Roan, West African Savannah Buffalo, amazing job. The dogs are used to hold the animal Korrigum, Central Kob, Sing-sing Waterbuck and in place while the hunter takes the shot. Without others may hunted in the Niwa and Vina hunting dogs, the Bongo would just keep running and areas.