Rangifer Tarandus) Hoof Suitable for Efficient Locomotion on Complex Grounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Foot and Mouth Disease Fastfact
Foot and Mouth Disease (FMD) What is foot and mouth How does FMD affect my Who should I contact if I disease and what causes it? animal? suspect FMD? Foot and mouth disease (FMD) is The most common signs of foot Contact your veterinarian a highly contagious viral disease of and mouth disease are fever and immediately. FMD is not currently cloven-hoofed (two-toed) animals (e.g., the formation of blisters, ulcers and found in the United States; suspicion cattle, pigs, sheep). FMD causes painful sores on the mouth, tongue, nose, of the disease requires immediate sores and blisters on the feet, mouth feet, and teats. Foot lesions occur in attention. the area of the coronary band and and teats of animals. between the toes. Infected cattle are How can I protect my animal Foot and mouth disease is a high depressed, reluctant to move, and from FMD? consequence livestock disease due to unwilling or unable to eat, which can To prevent the introduction of FMD, its potential for rapid spread, severe lead to decreased milk production, use strict biosecurity procedures on trade restrictions and the subsequent weight loss, and poor growth. Affected your farm. Isolate any new introductions economic impacts that would result. animals may also have nasal discharge or animal returning to the farm for The disease occurs in parts of Asia, and excessive salivation. Pigs often several weeks before introducing them have sore feet but less commonly into the herd. Minimize visitors on Africa, the Middle East and South develop mouth lesions. Sheep and your farm, especially those that have America, but has been eradicated goats show very mild, if any, signs of traveled from countries with FMD. -

AZA Ungulate TAG Midyear Meetings March 23-25, 2021 “Many Hooves, One Herd”
AZA Ungulate TAG Midyear Meetings March 23-25, 2021 “Many Hooves, One Herd” Tuesday March 23 - DAY 1 (All times are Eastern Standard Time) 11am-1pm Welcome, Overview, and Agenda for the Week – Moderator, Steve Metzler, Antelope, Cattle, Giraffid, and Camelid TAG Chair AZA Ungulate TAG Chair Briefings • Rhino TAG – Adam Eyres, TAG Chair, Fossil Rim Wildlife Center • Equid TAG – Tim Thier, TAG Chair, Saint Louis Zoo • Hippo, Peccary, Pig, and Tapir TAG – Martin Ramirez, TAG Chair, Woodland Park Zoo • Deer (Cervid/Tragulid) TAG – Michelle Hatwood, TAG Chair, Audubon Species Survival Center • Caprinae TAG – Gil Myers, TAG Chair, Smithsonian National Zoo • Antelope, Cattle, Giraffid, and Camelid (ACGC) TAG – Steve Metzler, TAG Chair, San Diego Zoo Wildlife Alliance 1pm-2:30pm Animal Program Leaders Meeting 3pm-5pm The Future of SSPs and How it May Impact the Ungulate TAGs and Collection Planning – Presentations and Discussion, Moderator, Steve Metzler • Panelists, Dave Powell, Animal Population Management Committee, Saint Louis Zoo and Ungulate TAG Chairs Wednesday March 24 – DAY 2 (All times are Eastern Standard Time) 11am-1pm Reports from the Field Part 1 – Moderators, Wendy Enright and RoxAnna Breitigan, The Living Desert Zoo and Gardens • Peninsular Pronghorn Conservation Project – Melodi Tayles, San Diego Zoo Wildlife Alliance • Large-antlered Muntjac CGF Grant – Michelle Hatwood, Audubon Species Survival Center • Action Indonesia Update – James Burton, IUCN Asian Wild Cattle Specialist Group • Saola Working Group activities - James Burton, -

Footrot in Sheep and Goats Lynn Pezzanite, Animal Sciences Student Dr
PURDUE EXTENSION Animal Sciences AS-596-W Footrot in Sheep and Goats Lynn Pezzanite, Animal Sciences Student Dr. Mike Neary, Small Ruminant Extension Specialist, Purdue University Terry Hutchens, Extension Goat Specialist, University of Kentucky Footrot is a costly disease in the sheep and goat warms to mud. Footrot is most prevalent and highly industry. Countless producers lose time and money contagious in wet, moist areas. When pastures have each year in an attempt to control it in their flock or been consistently wet with no dry spells there is a herd. If footrot becomes a problem, it takes much higher incidence of outbreaks. The ideal soil reservoir effort and labor to control symptoms and eliminate is high in moisture at temperatures between 50°F to it. However, footrot is a preventable disease with 70°F. attentive management. Symptoms Causes of Footrot Foot scald and footrot result in lameness, reduced Footrot is caused by the coexistence of two weight gain, decreased milk and wool production, gram-negative, anaerobic bacteria, Fusobacterium and decreased reproductive capabilities as severely necrophorum and Dichelobacter nodosus (also infected animals are reluctant to move in order to feed. referred to as Bacteroides nodosus). Several different Affected animals often carry the affected leg or lie strains of D. nodosus affect both sheep and goats, down for extended periods, rubbing off the wool/hair and can also be carried by cattle, deer, and horses. In on their flanks, brisket, and knees. These conditions general, sheep are affected more severely than goats. result in production losses, treatment and prevention The bacteria Fusobacterium necrophorum causes costs, premature culling, and reduced sale value of a common disease known as foot scald. -

The Analysis of Sea Turtle and Bovid Keratin Artefacts Using Drift
Archaeometry 49, 4 (2007) 685–698 doi: 10.1111/j.1475-4754.2007.00328.x BlackwellOxford,ARCHArchaeometry0003-813X©XXXORIGINALTheE. UniversityO. analysis Espinoza, UK Publishing ofofARTICLES seaB.Oxford, W. turtle LtdBaker 2007 and and bovid C. A.keratin THEBerry artefacts ANALYSIS OF SEA TURTLE AND BOVID KERATIN ARTEFACTS USING DRIFT SPECTROSCOPY AND DISCRIMINANT ANALYSIS* E. O. ESPINOZA† and B. W. BAKER US National Fish & Wildlife Forensics Laboratory, 1490 E. Main St, Ashland, OR 97520, USA and C. A. BERRY Department of Chemistry, Southern Oregon University, 1250 Siskiyou Blvd, Ashland, OR 97520, USA We investigated the utility of diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) for the analysis and identification of sea turtle (Family Cheloniidae) and bovid (Family Bovidae) keratins, commonly used to manufacture historic artefacts. Spectral libraries are helpful in determining the class of the material (i.e., keratin versus plastics), but do not allow for inferences about the species source of keratin. Mathematical post- processing of the spectra employing discriminant analysis provided a useful statistical tool to differentiate tortoiseshell from bovid horn keratin. All keratin standards used in this study (n = 35 Bovidae; n = 24 Cheloniidae) were correctly classified with the discriminant analysis. A resulting performance index of 95.7% shows that DRIFTS, combined with discriminant analysis, is a powerful quantitative technique for distinguishing sea turtle and bovid keratins commonly encountered in museum collections and the modern wildlife trade. KEYWORDS: KERATIN, DRIFT SPECTROSCOPY, DISCRIMINANT ANALYSIS, X-RAY FLUORESCENCE, SEA TURTLE, BOVID, TORTOISESHELL, HORN, WILDLIFE FORENSICS INTRODUCTION The keratinous scutes of sea turtles and horn sheaths of bovids have been used for centuries in artefact manufacture (Aikin 1840; Ritchie 1975). -

Mechanical Indices of Hoof Horn and Their Associations with Length Measurements and Lesion Scores
veterinary sciences Article Claw Characteristics of Culled Sows from Three Farrow-to-Finish Greek Farms. Part 2: Mechanical Indices of Hoof Horn and Their Associations with Length Measurements and Lesion Scores Sofia Chalvatzi 1,*, Georgios A. Papadopoulos 1 , Fotios Kroustallas 1 , Mihaela Cernat 2, Vassilis Skampardonis 2 , Christina Marouda 3, Vasileia Fotiadou 1, Vasileios Psychas 3, Theofilos Poutahidis 3, Leonidas Leontides 2 and Paschalis Fortomaris 1 1 Laboratory of Animal Husbandry, Faculty of Veterinary Medicine, School of Health Sciences, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] (G.A.P.); [email protected] (F.K.); [email protected] (V.F.); [email protected] (P.F.) 2 Department of Epidemiology, Biostatistics and Economics of Animal Production, School of Veterinary Medicine, University of Thessaly, 43132 Karditsa, Greece; [email protected] (M.C.); [email protected] (V.S.); [email protected] (L.L.) 3 Laboratory of Pathology, Faculty of Veterinary Medicine, School of Health Sciences, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] (C.M.); [email protected] (V.P.); [email protected] (T.P.) * Correspondence: [email protected] Citation: Chalvatzi, S.; Papadopoulos, G.A.; Kroustallas, F.; Abstract: The objective of the present study was to investigate the mechanical indices of hoof horn Cernat, M.; Skampardonis, V.; and their association with length measurements and lesion score. The feet of 185 culled sows from Marouda, C.; Fotiadou, V.; Psychas, V.; Poutahidis, T.; Leontides, L.; et al. three Greek farms (A: 57 sows; B: 64 sows; C: 64 sows) were used. A slice from the dorsal wall of each Claw Characteristics of Culled Sows claw was used to assess by a three-point bending test the Young’s modulus, yield stress and aximum from Three Farrow-to-Finish Greek stress values. -

FOI Summary, Thianil (Thiafentanil Oxalate), MIF 900-000
Date of Index Listing: June 16, 2016 FREEDOM OF INFORMATION SUMMARY ORIGINAL REQUEST FOR ADDITION TO THE INDEX OF LEGALLY MARKETED UNAPPROVED NEW ANIMAL DRUGS FOR MINOR SPECIES MIF 900-000 THIANIL (thiafentanil oxalate) Captive non-food-producing minor species hoof stock “For immobilization of captive minor species hoof stock excluding any member of a food- producing minor species such as deer, elk, or bison and any minor species animal that may become eligible for consumption by humans or food-producing animals.” Requested by: Wildlife Pharmaceuticals, Inc. Freedom of Information Summary MIF 900-000 TABLE OF CONTENTS I. GENERAL INFORMATION: ............................................................................. 1 II. EFFECTIVENESS AND TARGET ANIMAL SAFETY: .............................................. 1 A. Findings of the Qualified Expert Panel: ...................................................... 2 B. Literature Considered by the Qualified Expert Panel: ................................... 4 III. USER SAFETY: ............................................................................................. 6 IV. AGENCY CONCLUSIONS: .............................................................................. 7 A. Determination of Eligibility for Indexing: .................................................... 7 B. Qualified Expert Panel: ............................................................................ 7 C. Marketing Status: ................................................................................... 7 D. Exclusivity: -

Cattle, Sheep, Goats, and Horses: What’S the Difference for Working Rangelands?
ANR Publication 8524 | July 2015 http://anrcatalog.ucanr.edu ckr li UNDERSTANDING WORKING RANGELANDS Cattle, Sheep, Goats, and Horses: What’s the Difference for Working Rangelands? : rrunaway/F Photo INTRODUCTION omestic livestock commonly raised in California include STEPHANIE LARSON, University of California Cooperative Extension Dcattle (bovines), sheep (ovines), goats (caprines), and County Director and Livestock horses (equines). Cattle, sheep, and goats can be used for Range Management Advisor, the production of meat or dairy products, depending on Sonoma County; SHEILA BARRY, their breed and management. Horses are kept primarily for University of California Cooperative recreation and for use in ranch work. Each livestock species has Extension County Director and Livestock Range Management dissimilar feed and husbandry needs, interacts with humans Advisor, San Francisco Bay Area; differently, and has varying effects on the land and vegetation. and LISA BUSH, Rangeland Livestock affect land and vegetation in several interrelated ways, Consultant. including removal of leaves, stems, and other plant parts; removal or redistribution of nutrients; and mechanical impacts on soil and plants through trampling (Vallentine 1990). All of these factors vary by animal species, depending on dietary preference, digestive system, mouth anatomy, and Working rangelands are public or privately owned open space lands that are managed with livestock grazing and rancher stewardship. Photo: Josh Mazgelis/Flickr Their management contributes to the production of a variety of ecosystem services, including food, clean water, weed control, wildlife habitat, fire fuel reduction, carbon sequestration, pollination, aesthetic views, cultural heritage, recreational and educational opportunities, and open space conservation. ANR Publication 8524 | UNDERSTANDING WORKING RANGELANDS – Cattle, Sheep, Goats, and Horses: What’s the Difference for Working Rangelands | July 2015 | 2 animal size and weight. -

Milky Mammals Let’S Learn About Dairy Cows
MILKY MAMMALS LET’S LEARN ABOUT DAIRY COWS TEACHERS: Your field trip to the farm will create opportunities for your stu- dents to observe a variety of animals found on a farm. These observations inspire students to ask questions, collect and document data for journal entries and science investigations. Each hands- on discovery assists in connecting and enhancing Next Generation Science Standards. Grade Level/Range: Grades K-5 OBJECTIVE Students will carefully observe a variety of mammals found on a farm and understand the characteristics of mammals. Students will learn that Holstein cows are a breed of dairy animals Students will connect dairy products purchased at the grocery store to the farm/farmer that produced them Students will understand making health choices will grow a strong body DRESS UP LIKE A COW One student will volunteer to dress up like a cow. (Scientific name Bos Taurus) We start with a black and white fur (or hair) apron because the student will be turned into a Holstein cow. Holstein cattle are one of the 6 most common breeds of dairy cows in the United States. Dairy cattle are designed to produce more milk rather than muscle mass for the beef industry. Holstein’s cattle are mammals and most mammals have hair/fur which is very important in keeping animals warm. Mammals are warm-blooded and it is important for them to maintain their body temperature to be healthy. I modi- fied the statement on mammals because humans are the only mammals that regulate their internal tem- perature through sweating. Humans are considered hairless, having some hair on the head, axillae and groin. -

Exoskeleton Derivatives of Mammals Integumentaryderivative
Exoskeleton Derivatives of Mammals TIG The following points highlight the the seven main integumentary derivatives in mammals. The integumentary derivatives are: 1. Glands 2. Hair 3. Colour 4. Claws, Nails and Hoofs 5. Horns 6. Antlers 7. Epidermal Scales. IntegumentaryDerivative: 1.Glands: A large variety of epidermal glands is present in the skin of mammals. These glands are tubular or alveolar in nature and are always multicellular. The principal glands are: a. Sudorific or sweat glands: These are long and coiled tubular glands. The lower part of the gland lies embedded in the dermis and the upper part is constituted by a duct which opens to the outside through a pore. The distribution of sweat glands in different mammals is not uniform. In case of man the sweat glands are more numerous on palm, sole and arm pits. In cat, dog and rat sweat glands are more numerous on palm, sole and arm pits. In cat, dog and rat sweat glands are found in the sole of the feet. In rabbits the glands are found around the lips. The ruminants have sweat glands located on the muzzle and on the inter- digital fold of skin. In hippopotamus the pinna houses the sweat glands. Sweat glands are absent in Tachyglossus, Mus, Talpa, Cetacea and Sirenia. The sweat is watery in appearance but its colour is red in certain mammals like hippopotamus and Macropus. Function: The secretion of sweat glands is known as sweat. Sweat aids in removing nitrogenous wastes and helps in the regulation of body temperature. It is believed that the glands of Moll in the eye margin of man are modified sweat glands and similar is the case with ceruminous or wax- secreting glands in the external ear passage. -

Elk Hoof Disease in Southwest Washington
Elk Hoof Disease in Southwest Washington Kristin Mansfield DVM, MPVM Washington Department of Fish & Wildlife July 8, 2014 1 Photo by S. McCorquodale Wild Ungulate Hoof Diseases . Usually sporadic and many different causes . Below are photos from an elk, a moose, and a mule deer, each with a different hoof disease, all collected during Fall 2012 in Eastern WA 2 Examples of Deformed Elk Hooves 3 Prevalence and Distribution 2008-2009 4 Diagnostic Investigation Partners Assistance of Veterinary Samples Sent to Veterinary Personnel From: Diagnostic or Research Labs At: • WDFW • Washington State University • Washington State University • University of Idaho • University of Washington • Colorado State University • ODFW • University of Wyoming • Oregon State University • University of Liverpool (U.K.) • University of Wisconsin • USDA National Veterinary • Tufts University Services Laboratory • WSDA • USDA National Animal Disease Center • UC Davis 5 Diagnostic Investigation . Gross necropsy . Radiology . Histology . Parasitology . Virus isolation . Trace minerals . Routine bacteriology . Specialized bacteriology 6 Collections • March 2009 : adult cows 3 unaffected area -- East of I-5 5 affected area -- Lewis/Cowlitz Co. • Feb/Mar 2013: 9-10 month old calves 3 unaffected area -- Pacific County 4 unaffected area -- Yakima / Kittitas Co. 9 affected area -- Lewis / Cowlitz Co. • August 2013: 3 month old calves 2 unaffected area -- Grays Harbor Co. 5 affected area -- Lewis Co. • January 2014: 8 month old calves 2 unaffected area -- Kittitas Co. 9 affected area --Cowlitz, Wahkiakum, Pacific, Grays Harbor Co. 8 9 10 Conclusions • No significant underlying musculoskeletal or systemic disease • Disease is limited to the hooves 11 2013 – 9 month elk From tooth to hoof: treponemes in tissue-destructive diseases Treponemes: Spiral shaped bacteria, double membrane, flagella sandwiched between them. -
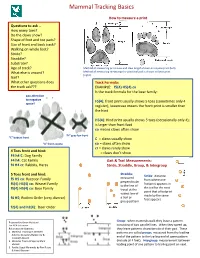
Mammal Tracking Basics
Mammal Tracking Basics How to measure a print Questions to ask … How many toes? Do the claws show? Shape of foot and toe pads? Size of front and back track? Walking on whole foot? Stride? Straddle? Substrate? Age of track? Method of measuring print sizes and claw length shown on coyote print (left). What else is around? Method of measuring metacarpal or proximal pad is shown on bear print (right). Gait? What other questions does Track Formula: the track ask??? EXAMPLE: f5(4) H5(4) co Is the track formula for the bear family: pay attention to negative F5(4): front print usually shows 5 toes (sometimes only 4 space! register), lowercase means the front print is smaller than the hind H5(4): Hind print usually shows 5 toes (occasionally only 4); is larger than front foot co means claws often show “H” grey fox front “C” bobcat front C = claws usually show “X” front coyote co = claws often show cr = claws rarely show 4 Toes front and hind: = claws don’t show F4 h4 C:Dog Family F4 h4: Cat Family Gait & Trail Measurements: f4 H4 cr: Rabbits, Hares Stride, Straddle, Group, & Intergroup Straddle: 5 Toes front and hind: Stride: distance measured f5 H5 co: Raccoon Family from where one perpendicular footprint appears in f5(4) H5(4) co: Weasel Family to the line of the trail to the next f5(4) H5(4) co: Bear Family travel at the point that a footprint widest line of made by the same a trail or f4 H5; Rodent Order (very diverse) foot appears group pattern f2(4) and h2(4): Deer Order Prepared by Gwen Heistand Group: when mammals walk they leave a pattern For ACR Education consisting of two parallel lines. -

Hoof Health — Whether the Weather Veterinary Services at Simplot Only One Or Maybe No Cases of for Trauma to the Feet and Pastures Are Wet Or Dry
Herd HEALTH The main one we deal with is high incidence of foot rot. On Fusobacterium necrophorum,” he says. the other hand, husbandry and These bacteria are almost environmental management always present in the environment. is a big part of the picture. If Signs of foot rot include swelling environmental issues are causing at the heel or between the claws, the problem, I would try to and the swelling sometimes breaks change those things. The vaccine open and drains. can be a good tool, but should be “The biggest thing in looked at as just one part of the preventing foot rot is managing total management.” the environment to reduce For treatment, he advises risks for foot rot. This means using an antibiotic with the eliminating mud as much as proper spectrum and durability possible, and reducing risks for of activity labeled for foot rot. trauma to the feet especially “LA-200 is an economical and between the toes. Rocky or frozen effective choice, especially if you conditions in which the skin may catch it early,” he says. be broken by sharp rocks or ice This is the key — catching it can predispose cattle to foot rot,” early, treating with the proper Raymond says. antibiotic and putting the animal “In the feed yard, we try where it’s clean and dry. to handle cattle with the least “Another antibiotic I often use amount of stress possible so they for foot rot is Excede. This factor are not scraping their toes on has a seven-day tissue level. We get concrete or getting abrasions on a longer duration of activity with the sides of their feet by being just one treatment.