Mediterranean Marine Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ANTIOXIDANT CAPACITY in the HEMOLYMPH of the MARINE ISOPOD PENTIDOTEA RESECATA by Leah E. Dann a THESIS Submitted to WALLA WALL
ANTIOXIDANT CAPACITY IN THE HEMOLYMPH OF THE MARINE ISOPOD PENTIDOTEA RESECATA By Leah E. Dann A THESIS submitted to WALLA WALLA UNIVERSITY in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE April 26, 2017 ABSTRACT The isopod Pentidotea resecata inhabits Zostera marina eelgrass beds. Examination of oxygen levels in a Z. marina bed indicated that P. resecata frequently experience hyperoxia and potential hypoxia reperfusion events in these beds, which may lead to enhanced reactive oxygen species (ROS) production and increased oxidative damage if the antioxidant defenses cannot sufficiently suppress these toxic oxygen intermediates. The total antioxidant capacity of P. resecata hemolymph was compared to that of Ligia pallasii, a semi-terrestrial isopod living in normoxic conditions, and to that of Pandalus danae, a shrimp that lives below the photic zone. The hypothesis was that P. resecata hemolymph would have stronger antioxidant defenses than the other crustaceans because this isopod faces a more hostile oxygen environment. LCMS analysis of P. resecata hemolymph confirmed the presence of antioxidants including pheophorbide a, lutein, and β-carotene, while L. pallasii hemolymph contained pheophorbide a and lutein but no β-carotene. Pandalus danae hemolymph had no carotenoids or pheophorbide. Although L. pallasii hemolymph was missing β-carotene, it had a significantly higher total antioxidant capacity than that of P. resecata. Hemolymph from P. danae had an intermediate antioxidant capacity even though it contained none of the antioxidants detected in the other species. The unexpected antioxidant activities among the species could be explained by differences in metabolic functions or environmental factors that were not examined in this study; or perhaps P. -

Colour Polymorphism and Genetic Variation in <Emphasis Type="Italic">Idotea Baltica</Emphasis> Populations
The Ecological Distribution of British Species of Idotea (Isopoda) STOR E. Naylor The Journal of Animal Ecology, Vol. 24, No. 2. (Nov., 1955), pp. 255-269. Stable URL: http://links.jstor.org/sici?sici=0021-8790%28195511%2924%3A2%3C255%3ATEDOBS%3E2.0.CO%3B2-%23 The Journal of Animal Ecology is currently published by British Ecological Society. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/joumals/briteco.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is an independent not-for-profit organization dedicated to creating and preserving a digital archive of scholarly journals. For more information regarding JSTOR, please contact [email protected]. http://www.j stor.org/ Tue Oct 3 15:24:28 2006 VOLUME 24, No. 2 NOVEMBER 1955 THE ECOLOGICAL DISTRIBUTION OF BRITISH SPECIES OF IDOTEA (ISOPODA) BY E. NAYLOR Marine Biological Station, Port Erin (With 4 Figures in the Text) INTRODUCTION Descriptions of the ecology of Idotea are often generalized, and there appears to be no comprehensive work on the habits of individual species. -

Appendix C - Invertebrate Population Attributes
APPENDIX C - INVERTEBRATE POPULATION ATTRIBUTES C1. Taxonomic list of megabenthic invertebrate species collected C2. Percent area of megabenthic invertebrate species by subpopulation C3. Abundance of megabenthic invertebrate species by subpopulation C4. Biomass of megabenthic invertebrate species by subpopulation C- 1 C1. Taxonomic list of megabenthic invertebrate species collected on the southern California shelf and upper slope at depths of 2-476m, July-October 2003. Taxon/Species Author Common Name PORIFERA CALCEREA --SCYCETTIDA Amphoriscidae Leucilla nuttingi (Urban 1902) urn sponge HEXACTINELLIDA --HEXACTINOSA Aphrocallistidae Aphrocallistes vastus Schulze 1887 cloud sponge DEMOSPONGIAE Porifera sp SD2 "sponge" Porifera sp SD4 "sponge" Porifera sp SD5 "sponge" Porifera sp SD15 "sponge" Porifera sp SD16 "sponge" --SPIROPHORIDA Tetillidae Tetilla arb de Laubenfels 1930 gray puffball sponge --HADROMERIDA Suberitidae Suberites suberea (Johnson 1842) hermitcrab sponge Tethyidae Tethya californiana (= aurantium ) de Laubenfels 1932 orange ball sponge CNIDARIA HYDROZOA --ATHECATAE Tubulariidae Tubularia crocea (L. Agassiz 1862) pink-mouth hydroid --THECATAE Aglaopheniidae Aglaophenia sp "hydroid" Plumulariidae Plumularia sp "seabristle" Sertulariidae Abietinaria sp "hydroid" --SIPHONOPHORA Rhodaliidae Dromalia alexandri Bigelow 1911 sea dandelion ANTHOZOA --ALCYONACEA Clavulariidae Telesto californica Kükenthal 1913 "soft coral" Telesto nuttingi Kükenthal 1913 "anemone" Gorgoniidae Adelogorgia phyllosclera Bayer 1958 orange gorgonian Eugorgia -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Idotea Granulosa Rathke, 1843
Idotea granulosa Rathke, 1843 AphiaID: 119044 ISÓPODE Animalia (Reino) >Arthropoda (Filo) >Crustacea (Subfilo) >Multicrustacea (Superclasse) >Malacostraca (Classe) >Eumalacostraca (Subclasse) > Peracarida (Superordem) > Isopoda (Ordem) > Valvifera (Subordem) > Idoteidae (Familia) Rainer Borcherding - Schutzstation Wattenmeer, via beachexplorer.org Estatuto de Conservação 1 Sinónimos Idotea cretaria Dahl, 1916 Referências additional source Schotte, M., B. F. Kensley, and S. Shilling. (1995-2017). World list of Marine, Freshwater and Terrestrial Crustacea Isopoda. National Museum of Natural History Smithsonian Institution: Washington D.C., USA [website archived on 2018-01-25]. [details] additional source Rappé, G. (1989). Annoted checklist of the marine and brackish-water Isopoda (Crustacea, Malacostraca) of Belgium, in: Wouters, K.; Baert, L. (Ed.) (1989). Proceedings of the Symposium “Invertebrates of Belgium”. pp. 165-168 [details] basis of record van der Land, J. (2001). Isopoda – excluding Epicaridea, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. 315-321 [details] additional source Muller, Y. (2004). Faune et flore du littoral du Nord, du Pas-de-Calais et de la Belgique: inventaire. [Coastal fauna and flora of the Nord, Pas-de-Calais and Belgium: inventory]. Commission Régionale de Biologie Région Nord Pas-de-Calais: France. 307 pp., available online at http://www.vliz.be/imisdocs/publications/145561.pdf [details] original description Rathke, H. (1843). Beiträge zur Fauna Norwegens. Nova Acta Academiae Caesareae Leopoldino-Carolinae Naturae Curiosorum, Breslau & Bonn. 20: 1-264c., available online at https://doi.org/10.5962/bhl.title.11613 [details] additional source Dyntaxa. -

Distribution and Inferred Evolutionary Characteristics of a Chimeric Ssdna Virus Associated with Intertidal Marine Isopods
Article Distribution and Inferred Evolutionary Characteristics of a Chimeric ssDNA Virus Associated with Intertidal Marine Isopods Kalia S. I. Bistolas 1,*, Ryan M. Besemer 2,3, Lars G. Rudstam 4 and Ian Hewson 1 1 Department of Microbiology, Cornell University, Ithaca, NY 14850, USA; [email protected] 2 New Visions Life Sciences, Boards of Cooperative Educational Services of New York State, Ithaca, NY 14850, USA; [email protected] 3 University of North Carolina at Wilmington, Wilmington, NC 28403, USA 4 Department of Natural Resources and the Cornell Biological Field Station, Cornell University, Bridgeport, NY 14850, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-607-255-0151 Received: 26 October 2017; Accepted: 23 November 2017; Published: 26 November 2017 Abstract: Aquatic invertebrates are common reservoirs of a rapidly expanding group of circular Rep-encoding ssDNA (CRESS-DNA) viruses. This study identified and explored the phylogenetic relationship between novel CRESS-DNA viral genotypes associated with Pacific intertidal isopods Idotea wosnesenskii, Idotea resecata, and Gnorimosphaeroma oregonensis. One genotype associated with I. wosnesenskii, IWaV278, shared sequence similarity and genomic features with Tombusviridae (ssRNA) and Circoviridae (ssDNA) genomes and was putatively assigned to the Cruciviridae clade comprising chimeric viruses. The complete genome of IWaV278 (3478 nt) was computationally completed, validated via Sanger sequencing, and exhibited sequence conservation and codon usage patterns analogous to other members of the Cruciviridae. Viral surveillance (qPCR) indicated that this virus was temporally transient (present in 2015, but not 2017), specific to I. wosnesenskii at a single collection site (Washington, DC, USA), more prevalent among male specimens, and frequently detected within exoskeletal structures. -

The Growth, Reproduction and Body Color Pattern of Cleantiella Isopus (Isopoda: Valvifera) in Hakodate Bay, Japan
CRUSTACEAN RESEARCH, NO. 41: 1–10, 2012 The growth, reproduction and body color pattern of Cleantiella isopus (Isopoda: Valvifera) in Hakodate Bay, Japan Tomohiro Takahashi and Seiji Goshima A b s t r a c t . — We studied the growth, cycling in the intertidal and subtidal areas. reproduction and body color pattern of the Most isopod studies focus on the life marine isopod Cleantiella isopus in Hakodate Bay, cycle, feeding habits and mate choice; Japan from May 2009 to July 2010. Individuals almost all are exclusively on European were collected every month and the sex, body species of the genus Idotea (Naylor, 1955a, length, color pattern, number of eggs per clutch b; Jormalainen et al., 1992). The breeding and developmental stage of embryos for ovigerous period and its length differ among species females were recorded. Five body color patterns or conspecific populations that occupy were identified in C. isopus at Hakodate Bay, and different habitats (Naylor, 1955b; Sheader, their composition was maintained throughout 1977; Salemaa, 1979; Healy & O’Neill, the year. Breeding females (guarded by a male 1984). Although about 20 species have been or carrying eggs) were observed in the field from reported in Japan, as far as we know, there February to August. Newly recruited individuals is only one study about the feeding habit of were first observed in July and grew to mature size by the next breeding season, indicating a Idotea ochotensis (Suzuki et al., 2002). Isopod lifespan of 13–15 months. Precopulatory mate species are common in northern Japan and guarding in which a male holds a female using his play an important role in the community of pereopods was observed. -
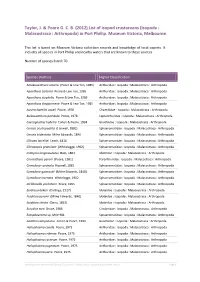
List of Isopod Crustaceans (Isopoda : Malacostraca : Arthropoda) in Port Phillip
Taylor, J. & Poore G. C. B. (2012) List of isopod crustaceans (Isopoda : Malacostraca : Arthropoda) in Port Phillip. Museum Victoria, Melbourne. This list is based on Museum Victoria collection records and knowledge of local experts. It includes all species in Port Phillip and nearby waters that are known to these sources. Number of species listed: 70. Species (Author) Higher Classification Amakusanthura olearia (Poore & Lew Ton, 1985) Anthuridae : Isopoda : Malacostraca : Arthropoda Apanthura isotoma Poore & Lew Ton, 1985 Anthuridae : Isopoda : Malacostraca : Arthropoda Apanthura styphelia Poore & Lew Ton, 1985 Anthuridae : Isopoda : Malacostraca : Arthropoda Apanthura thryptomene Poore & Lew Ton, 1985 Anthuridae : Isopoda : Malacostraca : Arthropoda Austrochaetilia capeli Poore, 1978 Chaetiliidae : Isopoda : Malacostraca : Arthropoda Bullowanthura pambula Poore, 1978 Leptanthuridae : Isopoda : Malacostraca : Arthropoda Caecognathia huberia Cohen & Poore, 1994 Gnathiidae : Isopoda : Malacostraca : Arthropoda Cerceis acuticaudata (Haswell, 1881) Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Cerceis tridentata Milne Edwards, 1840 Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Cilicaea latreillei Leach, 1818 Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Cilicaeopsis granulata (Whitelegge, 1902) Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Crabyzos longicaudatus Bate, 1863 Idoteidae : Isopoda : Malacostraca : Arthropoda Cruranthura peroni (Poore, 1981) Paranthuridae : Isopoda : Malacostraca : Arthropoda Cymodoce -

Idotea Wosnesenskii Class: Malacostraca Order: Isopoda Family: Idoteidae
Phylum: Arthropoda, Crustacea Idotea wosnesenskii Class: Malacostraca Order: Isopoda Family: Idoteidae Taxonomy: The genus Idotea was described pod”). Posterior to the pereon is the pleon, or by Fabricius in 1798, and although originally abdomen, with six segments, the last of which spelled Idotea, several authors adopted the is fused with the telson (the pleotelson) (see spelling Idothea, since then. The genus Plate 231, Brusca et al. 2007). The Isopoda Pentidotea was described by Richardson in can be divided into two groups: ancestral 1905 and was reduced to subgeneric level by (“short-tailed”) groups (i.e. suborders) that Menzies in 1950. The two subgenera (or have short telsons and derived (“long-tailed”) genera), Pentidotea and Idotea differ by the groups with long telsons. Valviferan articles on maxilliped palps, the former with (including the Idoteidae) isopods have an five and the latter with four (Miller and Lee elongated telson (Fig. 73, Ricketts and Calvin 1970), but are not always currently 1952). Idotea wosnesenskii individuals are recognized (Rafi and Laubitz 1990). robust, not tapered, elongate and depressed Furthermore, this character may vary with age (see Fig. 62, Ricketts and Calvin 1952). and other characters may reveal more Cephalon: Wider than long, with frontal concrete differences to define the two (Poore margin slightly concave (Miller 1968) and and Ton 1993). Thus synonyms for I. posterior portion somewhat wider than wosnesenskii include, Idothea wosnesenskii, anterior portion (Richarson 1905). Head Pentidotea wosnesenskii and Idotea narrower than pleon (Schultz 1969). First Pentidotea wosnesenskii. We follow the most thoracic segment fused with head (Isopoda, recent intertidal guide for the northeast Pacific Brusca et al. -

Species Differentiation in Synidotea (Isopoda: Idoteidae) and Recognition of Introduced Marine Species: a Reply to Chapman and Carlton
JOURNAL OF CRUSTACEAN BIOLOGY, 16(2): 384-394, 1996 SPECIES DIFFERENTIATION IN SYNIDOTEA (ISOPODA: IDOTEIDAE) AND RECOGNITION OF INTRODUCED MARINE SPECIES: A REPLY TO CHAPMAN AND CARLTON Gary C. B. Poore ABSTRACT Nominal species of the idoteid isopod genus Synidotea from Japan (S. laevidorsalis), western U.S.A. (5. laticauda), South Africa (S. hirtipes), and Australia (S. keablei and S. grisea) are shown to be morphologically distinct. Others probably are also. Contrary to the views of Chap man and Carlton (1991, 1994), the Japanese species has not been widely distributed by shipping. The Australian species fail several of Chapman and Carlton's attributed of introduced species: their recent discovery and restricted distribution are anticipated in a poorly explored fauna, there is no evidence of postintroduction range extension, no known human mechanisms of introduction exist, they are not associated with known introductions, nor with altered environ ments, and exotic evolutionary origin cannot be assessed while the phylogeny of the genus is not known. The species in the western U.S.A. is ecologically as well as morphologically distinct, being estuarine rather than marine. A record of S. laevidorsalis from the Gironde estuary, France, is, in fact, of S. laticauda and therefore an introduction from the U.S.A. rather than from Japan. This study demonstrates the importance of careful taxonomic analysis before it is concluded that marine species are introduced. Introductions of marine organisms from are reported from the Indo-West Pacific re one coast to another have been long known. gion, from the central Pacific, southern In Australia, the occurrence of the Euro Australia, the Indian Ocean, and South Af pean crab Carcinus maenas (L.) in Port rica. -

Présence Du Crustacé Idotea Metallica (Isopoda : Valvifera) Dans Le Parc National De Port-Cros (France, Méditerranée)
Sci. Rep. Port-Cros natl. Park, Fr., 25: 173-187 (2011) Présence du crustacé Idotea metallica (Isopoda : Valvifera) dans le Parc national de Port-Cros (France, Méditerranée) Pierre-Yves NOËL1*, Chantal JOMARD2 1 UMR CNRS n° 7208 Biologie des Organismes Marins et Ecosystèmes, Département Milieux et Peuplements Aquatiques, Muséum national d’Histoire naturelle, 61 rue Buffon, F-75005 Paris, France. 2 Parc national de Port-Cros, Allée du Castel Sainte Claire, BP 70220, 83406 Hyères Cedex, France. * Contact : [email protected] Résumé. Des spécimens du crustacé isopode Idotea metallica ont été observés à plusieurs reprises sur des macro-déchets flottants dans les eaux du Parc national de Port-Cros en 2010. Un récapitulatif bibliographique détaillé des localités où cette espèce a déjà été observée a été effectué, ainsi que le relevé des localités (souvent inédites) de récolte des spécimens de cette espèce qui existent dans les collections du Muséum national d’Histoire naturelle de Paris (une quinzaine de stations entre 1815 et 1972). D’après ces données, il apparaît que l’espèce n’est ni introduite, ni cryptogène. Cette espèce neustonique et cosmopolite se rencontre sur les objets naturels flottants et semble de plus en plus fréquente sur les déchets d’origine anthropique, y compris sur des composés pétroliers. L’éventualité d’un transfert de polluants de cette proie vers ses prédateurs (oiseaux marins, poissons pélagiques) est évoquée. Abstract. Occurrence of the crustacean Idotea metallica (Isopoda: Valvifera) in Port- Cros national park (France, Mediterranean). Idotea metallica was recorded several times in 2010 on floating macro-detritus of Port-Cros national park. -

The Occurrence of Idotea Metallica Bosc in British Waters
J. mar. bioI. Ass. U.K. (1957) 36, 599-602 599 Printed in Great Britain THE OCCURRENCE OF IDOTEA METALLICA BOSC IN BRITISH WATERS By E. NAYLOR University College of Swansea (Text-figs. I and 2) Idotea metallica Bose occurs occasionally in plankton from waters off the west coast of Britain, but since it does not seem to be a British resident it was excluded from a recent review of the genus Idotea (Naylor, 1955a). Accounts of the distribution of I. metallica seldom distinguish between occasional records and resident breeding localities, and this note attempts to explain its occasional occurrence in Britain. I am grateful to Dr J. H. Fraser, Dr K. M. Rae and Mr G. M. Spooner for the loan of material, and to Dr I. Gordon for facilities at the British Museum. Prof. E. W. Knight-Jones and Dr R. J. Menzies have kindly criticized the manuscript. SPECIFIC CHARACTERS (Fig. I) Body oblong; length about three times the width, except in females which are relatively much broader. Cephalon about If times as broad as long; anterior border concave, posterior border less so; marked transverse sinuous furrow behind the eyes; eyes large. Antennules hardly extending beyond the third joint of the antennal peduncle; first and second joints expanded, others fairly robust. Aesthetascs in pairs, numbering up to 20 or more in males; fewer in females. Antenna robust, flagellum shorter than peduncle and about one-sixth the length of the body; flagellum segments numbering up to about 10 in males and 8 in females; terminal style blunt, one quarter to one-sixth the length of the subterminal segment.