Reproducibility of Clinical Research in Critical Care: a Scoping Review Daniel J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

Meta-Analysis of Proportions
NCSS Statistical Software NCSS.com Chapter 456 Meta-Analysis of Proportions Introduction This module performs a meta-analysis of a set of two-group, binary-event studies. These studies have a treatment group (arm) and a control group. The results of each study may be summarized as counts in a 2-by-2 table. The program provides a complete set of numeric reports and plots to allow the investigation and presentation of the studies. The plots include the forest plot, radial plot, and L’Abbe plot. Both fixed-, and random-, effects models are available for analysis. Meta-Analysis refers to methods for the systematic review of a set of individual studies with the aim to combine their results. Meta-analysis has become popular for a number of reasons: 1. The adoption of evidence-based medicine which requires that all reliable information is considered. 2. The desire to avoid narrative reviews which are often misleading. 3. The desire to interpret the large number of studies that may have been conducted about a specific treatment. 4. The desire to increase the statistical power of the results be combining many small-size studies. The goals of meta-analysis may be summarized as follows. A meta-analysis seeks to systematically review all pertinent evidence, provide quantitative summaries, integrate results across studies, and provide an overall interpretation of these studies. We have found many books and articles on meta-analysis. In this chapter, we briefly summarize the information in Sutton et al (2000) and Thompson (1998). Refer to those sources for more details about how to conduct a meta- analysis. -

Reproducibility, Replicability, and Generalization in the Social, Behavioral, and Economic Sciences
REPRODUCIBILITY, REPLICABILITY, AND GENERALIZATION IN THE SOCIAL, BEHAVIORAL, AND ECONOMIC SCIENCES Report of the Subcommittee on Replicability in Science of the SBE Advisory Committee to the National Science Foundation 13 May 2015 Presentation at SBE AC Spring Meeting by K. Bollen. Report of the Subcommittee on Replicability in Science Advisory Committee to the NSF SBE Directorate Subcommittee Members: Kenneth Bollen (University of North Carolina at Chapel Hill, Cochair) John T. Cacioppo (University of Chicago, Cochair) Robert M. Kaplan (Agency for Healthcare Research and Quality) Jon A. Krosnick (Stanford University) James L. Olds (George Mason University) Staff Assistance Heather Dean (National Science Foundation) TOPICS I. Background II. Subcommittee Report III. Definitions IV. Recommendations V. Final Comments Background • Spring, 2013 o NSF SBE Advisory Committee establishes subcommittee on how SBE can promote robust research practices • Summer & Fall 2013 o Subcommittee proposal for workshop on “Robust Research in the Social, Behavioral, and Economic Sciences.” • February 20-21, 2014 o Workshop convened o Participants drawn from variety of universities, funding agencies, scientific associations, and journals o Cover broad range of issues from extent and cause of problems to possible solutions o Details are in the appendix of the circulated report • Post-workshop period o Document & digest workshop content o Discuss and propose recommendations o Complete report Subcommittee Report • DEFINITIONS o No consensus in science on the meanings -
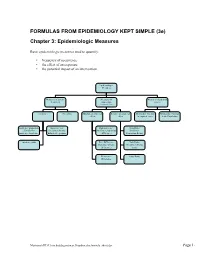
FORMULAS from EPIDEMIOLOGY KEPT SIMPLE (3E) Chapter 3: Epidemiologic Measures
FORMULAS FROM EPIDEMIOLOGY KEPT SIMPLE (3e) Chapter 3: Epidemiologic Measures Basic epidemiologic measures used to quantify: • frequency of occurrence • the effect of an exposure • the potential impact of an intervention. Epidemiologic Measures Measures of disease Measures of Measures of potential frequency association impact (“Measures of Effect”) Incidence Prevalence Absolute measures of Relative measures of Attributable Fraction Attributable Fraction effect effect in exposed cases in the Population Incidence proportion Incidence rate Risk difference Risk Ratio (Cumulative (incidence density, (Incidence proportion (Incidence Incidence, Incidence hazard rate, person- difference) Proportion Ratio) Risk) time rate) Incidence odds Rate Difference Rate Ratio (Incidence density (Incidence density difference) ratio) Prevalence Odds Ratio Difference Macintosh HD:Users:buddygerstman:Dropbox:eks:formula_sheet.doc Page 1 of 7 3.1 Measures of Disease Frequency No. of onsets Incidence Proportion = No. at risk at beginning of follow-up • Also called risk, average risk, and cumulative incidence. • Can be measured in cohorts (closed populations) only. • Requires follow-up of individuals. No. of onsets Incidence Rate = ∑person-time • Also called incidence density and average hazard. • When disease is rare (incidence proportion < 5%), incidence rate ≈ incidence proportion. • In cohorts (closed populations), it is best to sum individual person-time longitudinally. It can also be estimated as Σperson-time ≈ (average population size) × (duration of follow-up). Actuarial adjustments may be needed when the disease outcome is not rare. • In an open populations, Σperson-time ≈ (average population size) × (duration of follow-up). Examples of incidence rates in open populations include: births Crude birth rate (per m) = × m mid-year population size deaths Crude mortality rate (per m) = × m mid-year population size deaths < 1 year of age Infant mortality rate (per m) = × m live births No. -

CHAPTER 3 Comparing Disease Frequencies
© Smartboy10/DigitalVision Vectors/Getty Images CHAPTER 3 Comparing Disease Frequencies LEARNING OBJECTIVES By the end of this chapter the reader will be able to: ■ Organize disease frequency data into a two-by-two table. ■ Describe and calculate absolute and relative measures of comparison, including rate/risk difference, population rate/risk difference, attributable proportion among the exposed and the total population and rate/risk ratio. ■ Verbally interpret each absolute and relative measure of comparison. ■ Describe the purpose of standardization and calculate directly standardized rates. ▸ Introduction Measures of disease frequency are the building blocks epidemiologists use to assess the effects of a disease on a population. Comparing measures of disease frequency organizes these building blocks in a meaningful way that allows one to describe the relationship between a characteristic and a disease and to assess the public health effect of the exposure. Disease frequencies can be compared between different populations or between subgroups within a population. For example, one might be interested in comparing disease frequencies between residents of France and the United States or between subgroups within the U.S. population according to demographic characteristics, such as race, gender, or socio- economic status; personal habits, such as alcoholic beverage consump- tion or cigarette smoking; and environmental factors, such as the level of air pollution. For example, one might compare incidence rates of coronary heart disease between residents of France and those of the United States or 57 58 Chapter 3 Comparing Disease Frequencies among U.S. men and women, Blacks and Whites, alcohol drinkers and nondrinkers, and areas with high and low pollution levels. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Outcome Reporting Bias in COVID-19 Mrna Vaccine Clinical Trials
medicina Perspective Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials Ronald B. Brown School of Public Health and Health Systems, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] Abstract: Relative risk reduction and absolute risk reduction measures in the evaluation of clinical trial data are poorly understood by health professionals and the public. The absence of reported absolute risk reduction in COVID-19 vaccine clinical trials can lead to outcome reporting bias that affects the interpretation of vaccine efficacy. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in Pfzier/BioNTech and Moderna COVID-19 mRNA vaccine clinical trials. Based on data reported by the manufacturer for Pfzier/BioNTech vaccine BNT162b2, this critical appraisal shows: relative risk reduction, 95.1%; 95% CI, 90.0% to 97.6%; p = 0.016; absolute risk reduction, 0.7%; 95% CI, 0.59% to 0.83%; p < 0.000. For the Moderna vaccine mRNA-1273, the appraisal shows: relative risk reduction, 94.1%; 95% CI, 89.1% to 96.8%; p = 0.004; absolute risk reduction, 1.1%; 95% CI, 0.97% to 1.32%; p < 0.000. Unreported absolute risk reduction measures of 0.7% and 1.1% for the Pfzier/BioNTech and Moderna vaccines, respectively, are very much lower than the reported relative risk reduction measures. Reporting absolute risk reduction measures is essential to prevent outcome reporting bias in evaluation of COVID-19 vaccine efficacy. Keywords: mRNA vaccine; COVID-19 vaccine; vaccine efficacy; relative risk reduction; absolute risk reduction; number needed to vaccinate; outcome reporting bias; clinical epidemiology; critical appraisal; evidence-based medicine Citation: Brown, R.B. -

Copyright 2009, the Johns Hopkins University and John Mcgready. All Rights Reserved
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this site. Copyright 2009, The Johns Hopkins University and John McGready. All rights reserved. Use of these materials permitted only in accordance with license rights granted. Materials provided “AS IS”; no representations or warranties provided. User assumes all responsibility for use, and all liability related thereto, and must independently review all materials for accuracy and efficacy. May contain materials owned by others. User is responsible for obtaining permissions for use from third parties as needed. Section F Measures of Association: Risk Difference, Relative Risk, and the Odds Ratio Risk Difference Risk difference (attributable risk)—difference in proportions - Sample (estimated) risk difference - Example: the difference in risk of HIV for children born to HIV+ mothers taking AZT relative to HIV+ mothers taking placebo 3 Risk Difference From csi command, with 95% CI 4 Risk Difference Interpretation, sample estimate - If AZT was given to 1,000 HIV infected pregnant women, this would reduce the number of HIV positive infants by 150 (relative to the number of HIV positive infants born to 1,000 women not treated with AZT) Interpretation 95% CI - Study results suggest that the reduction in HIV positive births from 1,000 HIV positive pregnant women treated with AZT could range from 75 to 220 fewer than the number occurring if the -

Certification Accredited Schools List
= Academic Clinical Research Programs Which Currently Meet Waiver Requirements for the Academy of Clinical Research Professional (Updated October 15, 2015) This list includes academic clinical research programs which, as of the above date, have been evaluated and found to meet the current waiver requirements established by the Academy of Clinical Research Professionals (Academy) Board of Trustees. Programs that meet the waiver requirements allow a candidate to waive 1,500 hours of hands-on experience performing the Essential Duties of the program to which the candidate has made application. This list is not to be considered exhaustive and is not designed to represent all the academic offerings available. Appearance on this list is not to be construed as an endorsement of its content or format by the Academy or a guarantee that a candidate will be eligible for certification. All individuals interested in enrolling in a program of study should evaluate any program on the basis of his or her personal needs. (* - programs marked with an asterisk MAY be accepted but acceptance will be dependent on the complete listing of courses completed) Individuals seeking additional information about each program should contact the institution directly. Academic Institution Program Name ( Click Title for Web Navigation) Academy of Applied Pharmaceutical Sciences Clinical Research Diploma Program American University of Health Sciences Master of Science in Clinical Research Anoka-Ramsey Community College Clinical Research Professional - Certificate Graduate -

Placebo-Controlled Trials of New Drugs: Ethical Considerations
Reviews/Commentaries/Position Statements COMMENTARY Placebo-Controlled Trials of New Drugs: Ethical Considerations DAVID ORENTLICHER, MD, JD als, placebo controls are not appropriate when patients’ health would be placed at significant risk (8–10). A placebo- controlled study for a new hair- uch controversy exists regarding sufficiently more effective than placebo to thickening agent could be justified; a the ethics of placebo-controlled justify its use. Finally, not all established placebo-controlled study for patients M trials in which an experimental therapies have been shown to be superior with moderate or severe hypertension therapy will compete with an already es- to placebo. If newer drugs are compared would not be acceptable (11). Similarly, if tablished treatment (or treatments). In with the unproven existing therapies, an illness causes problems when it goes such cases, argue critics, patients in the then patients may continue to receive untreated for a long period of time, a 52- control arm of the study should receive an drugs that are harmful without being week study with a placebo control is accepted therapy rather than a placebo. helpful. much more difficult to justify than a By using an active and effective drug, the Moreover, say proponents of placebo 6-week study (12). control patients would not be placed at controls, patients can be protected from When David S.H. Bell (13) explains risk for deterioration of their disease, and harm by “escape” criteria, which call for why placebo controls are unacceptable the study would generate more meaning- withdrawal from the trial if the patient for new drugs to treat type 2 diabetes, he ful results for physicians. -

Generating Real-World Evidence by Strengthening Real-World Data Sources
Generating Real-World Evidence by Strengthening Real-World Data Sources Using “real-world evidence” to bring new “Every day, health care professionals are updating patients’ treatments to patients as part of the 21st electronic health records with data on clinical outcomes Century Cures Act (the “Cures Act”) is a key resulting from medical interventions used in routine clinical priority for the Department of Health and practice. As our experience with new medical products Human Services (HHS). Specifically, the Cures expands, our knowledge about how to best maximize their Act places focus on the use of real-world data benefits and minimize potential risks sharpens with each data to support regulatory decision-making, including point we gather. Every clinical use of a product produces data the approval of new indications for existing that can help better inform us about its safety and efficacy.” drugs in order to make drug development faster jacqueline corrigan-curay, md, jd and more efficient. As such, the Cures Act director of the office of medical policy in fda’s center for drug evaluation and research has tasked the Food and Drug Administration (FDA) to develop a framework and guidance for evaluating real-world evidence in the context of drug regulation.1 Under the FDA’s framework, real-world evidence (RWE) is generated by different study designs or analyses, including but not limited to, randomized trials like large simple trials, pragmatic trials, and observational studies. RWE is the clinical evidence about the use, potential benefits, and potential risks of a medical product based on an analysis of real-world data (RWD). -

Metabolic Sensor Governing Bacterial Virulence in Staphylococcus Aureus
Metabolic sensor governing bacterial virulence in PNAS PLUS Staphylococcus aureus Yue Dinga, Xing Liub, Feifei Chena, Hongxia Dia, Bin Xua, Lu Zhouc, Xin Dengd,e, Min Wuf, Cai-Guang Yangb,1, and Lefu Lana,1 aDepartment of Molecular Pharmacology and bChinese Academy of Sciences Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; cDepartment of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai 201203, China; dDepartment of Chemistry and eInstitute for Biophysical Dynamics, The University of Chicago, Chicago, IL 60637; and fDepartment of Basic Sciences University of North Dakota School of Medicine and Health Sciences, Grand Forks, ND 58203 Edited by Richard P. Novick, New York University School of Medicine, New York, NY, and approved October 14, 2014 (received for review June 13, 2014) An effective metabolism is essential to all living organisms, in- To survive and replicate efficiently in the host, S. aureus has cluding the important human pathogen Staphylococcus aureus.To developed exquisite mechanisms for scavenging nutrients and establish successful infection, S. aureus must scavenge nutrients adjusting its metabolism to maintain growth while also coping with and coordinate its metabolism for proliferation. Meanwhile, it also stress (6, 11). On the other hand, S. aureus produces a wide array must produce an array of virulence factors to interfere with host of virulence factors to evade host immune defenses and to derive defenses. However, the ways in which S. aureus ties its metabolic nutrition either parasitically or destructively from the host during state to its virulence regulation remain largely unknown. Here we infections (6).