Dietary Characteristics of Juvenile Trout and Char in Seasonally Inundated Stream Segments in Ross Lake, Washington
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

108Th Congress of the United States WASHINGTON CANADA
108th Congress of the United States WASHINGTON CANADA 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 33 3 3 3 333333 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 Osoyoos Nooksack 3 3 3 3 3 3 3 Lake Trust Land 3 3 3 3 3 3 3 3 3 3 3 r 3 e 3 3 v 3 3 i 3 R 3 Birch 3 ck 3 Ross Strait of Georgia 3 sa 3 3 Bay ok 3 o 3 N 3 3 Nooksack 3 Lake 3 3 3 3 Trust Land 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 North Cascades 3 3 3 3 3 3 3 3 3 3 3 National Park 3 WHATCOM 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 PEND 3 3 3 3 3 3 3 3 3 3 3 3 3 Lummi Nooksack Res 3 3 3 3 3 3 3 3 Res 3 3 3 OREILLE 3 3 3 3 3 3 3 Bellingham 3 3 3 Bay 3 33 3 3 Pend Oreille River 3 3 3 3 3 3 3 3 3 3 3 Bellingham 3 3 3 3 3 3 3 3 3 3 3 3 3 Ross Lake 3 3 Cowlitz 3 3 3 3 3 3 3 3 3 3 3 3 3 NRA 3 3 3 3 3 3 3 3 3 3 3 3 Bay 3 3 3 3 3 3 3 Okanogan River 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 Samish 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 TDSA 3 3 3 3 3 3 3 3 3 3 3 Samish 3 3 3 3 3 3 3 3 Rosario Strait Bay 3 3 FERRY 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 Upper Skagit Res 3 SAN JUAN 3 Haro Strait 3 North Cascades 3 3 3 3 3 3 3 National Park 3 OKANOGAN Upper Skagit Res 3 3 3 3 3 3 3 3 3 Skagit River 3 3 3 3 3 3 3 Padilla 3 3 3 3 Griffin Bay 3 3 3 3 3 3 3 Bay 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 US Coast Guard Station 3 3 3 SKAGIT 3 3 3 3 3 3 3 Neah Bay 3 3 3 3 3 3 3 3 3 3 3 STEVENS 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 -

The Skagit-High Ross Controversy: Negotiation and Settlement
Volume 26 Issue 2 U.S. - Canada Transboundary Resource Issues Spring 1986 The Skagit-High Ross Controversy: Negotiation and Settlement Jackie Krolopp Kirn Marion E. Marts Recommended Citation Jackie K. Kirn & Marion E. Marts, The Skagit-High Ross Controversy: Negotiation and Settlement, 26 Nat. Resources J. 261 (1986). Available at: https://digitalrepository.unm.edu/nrj/vol26/iss2/6 This Article is brought to you for free and open access by the Law Journals at UNM Digital Repository. It has been accepted for inclusion in Natural Resources Journal by an authorized editor of UNM Digital Repository. For more information, please contact [email protected], [email protected], [email protected]. JACKIE KROLOPP KIRN* and MARION E. MARTS** The Skagit-High Ross Controversy: Negotiation and Settlement SETTING AND BACKGROUND The Skagit River is a short but powerful stream which rises in the mountains of southwestern British Columbia, cuts through the northern Cascades in a spectacular and once-remote mountain gorge, and empties into Puget Sound approximately sixty miles north of Seattle. The beautiful mountain scenery of the heavily glaciated north Cascades was formally recognized in the United States by the creation of the North Cascades National Park and the Ross Lake National Recreation Area in 1968, and earlier in British Columbia by creation of the E.C. Manning Provincial Park. The Ross Lake Recreation Area covers the narrow valley of the upper Skagit River in Washington and portions of several tributary valleys. It was created as a political and, to environmentalists who wanted national park status for the entire area, controversial, compromise which accom- modated the city of Seattle's Skagit River Project and the then-planned North Cascades Highway. -

The Damnation of a Dam : the High Ross Dam Controversy
THE DAMYIATION OF A DAM: TIIE HIGH ROSS DAM CONTROVERSY TERRY ALLAN SIblMONS A. B., University of California, Santa Cruz, 1968 A THESIS SUBIUTTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in the Department of Geography SIMON FRASER UNIVERSITY May 1974 All rights reserved. This thesis may not b? reproduced in whole or in part, by photocopy or other means, without permission of the author. APPROVAL Name: Terry Allan Simmons Degree: Master of Arts Title of Thesis: The Damnation of a Dam: The High Ross Dam Controversy Examining Committee: Chairman: F. F. Cunningham 4 E.. Gibson Seni Supervisor / /( L. J. Evendon / I. K. Fox ernal Examiner Professor School of Community and Regional Planning University of British Columbia PARTIAL COPYRIGHT LICENSE I hereby grant to Simon Fraser University rhe righc to lcnd my thesis or dissertation (the title of which is shown below) to users of the Simon Fraser University Library, and to make partial or single copies only for such users or in response to a request from the library of any other university, or other educational institution, on its own behalf or for one of its users. I further agree that permission for multiple copying of this thesis for scholarly purposes may be granted by me or the Dean of Graduate Studies. It is understood that copying or publication of this thesis for financial gain shall not be allowed ' without my written permission. Title of' ~hesis /mqqmkm: The Damnation nf a nam. ~m -Author: / " (signature ) Terrv A. S.imrnonze (name ) July 22, 1974 (date) ABSTRACT In 1967, after nearly fifty years of preparation, inter- national negotiations concerning the construction of the High Ross Dan1 on the Skagit River were concluded between the Province of British Columbia and the City of Seattle. -
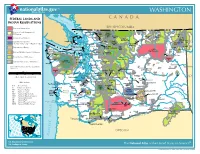
WASHINGTON Where We Are
nationalatlas.gov TM WASHINGTON Where We Are FEDERAL LANDS AND CANADA Str ait INDIAN RESERVATIONS of Ge org ia BRITISH COLUMBIA Bureau of Indian Affairs Blaine Mount Baker Bureau of Land Management / Oroville Helicopter Training Mount North Wilderness Area Baker Lummi IR Cascades Colville Colville NF NP Ross Lake Okanogan San Juan Islands National Forest R NF Bureau of Reclamation Bellingham NRA NF Cusick NWR R Okanogan a S Conconully Republic i Survival tr National Forest Lake b Training Kaniksu Department of Defense ai San Juan Island Mount Baker North t o Anacortes Skagit NRA m Site NF f NHP Cascades Little Pend Oreille (includes Army Corps of Engineers lakes) Jua Swinomish IR Conconully u n NP l NWR de Reservoir Omak Makah F o uca Whidbey Island NAS Mount Vernon Mount Baker Lake Kalispel IR Department of Energy IR Helicopter Training Area Chelan C Colville Flattery Rocks NWR Naval NRA Colville NF Kaniksu Ebey's Landing NHR Reservation Ozette IR Lower Elwha IR Indian Reservation NF Fish and Wildlife Service / Wilderness Lakewood Mount Baker Newport Olympic Port Angeles Dungeness Olympic Protection Island National Forest Lake Roosevelt NP NWR Tulalip IR NF NWR National Recreation Area Forest Service / Wilderness Everett Forks Olympic NF Wenatchee Grand Coulee Spokane Quileute IR National Forest Olympic Port Gamble IR IR National Park Service / Wilderness National Park d Lynnwood Snoqualmie Port Madison IR n Chelan Hoh IR u NF Banks Wilbur o S Lake Spokane t Seattle Some small sites are not shown, especially in R e Opportunity t g ul Bremerton Leavenworth Coulee City u Bellevue urban areas. -

EDR Inc.EDR Inc
8 40 1 6 0 0 8 0 11 2 5 1 k 40 V 0 4 8 0 40 8 0 80 0 2 0 4 0 4 1 8 0 40 3 46 0 4 40 160 1 2 2 120 80 8 0 160 0 2 0 0 2 4 0 40 2 8 0 3 2 0 2 8 3 6 0 0 0 0 8 6 1 0 0 8 0 0 2 2 2 3 0 0 240 4 0 0 2 0 1 4 0 0 6 6 3 1 320 80 0 2 1 0 4 2 0 0 8 8 2 0 0 2 0 0 4 0 2 3 0 6 1 0 4 2 0 2 1 0 0 2 EDR Inc.EDR Inc.EDR Inc. 40 0 40 EDR Inc.EDR Inc.EDR Inc. MAP FINDINGS SUMMARY Search Distance Target Total Database(Miles) Property < 1/8 1/8 - 1/4 1/4 - 1/2 1/2 - 1 > 1 Plotted STANDARD ENVIRONMENTAL RECORDS Federal NPL site list NPL 1.000 0 0 0 0 NR 0 Proposed NPL 1.000 0 0 0 0 NR 0 NPL LIENS TP NR NR NR NR NR 0 Federal Delisted NPL site list Delisted NPL 1.000 0 0 0 0 NR 0 Federal CERCLIS list FEDERAL FACILITY 0.500 0 0 0 NR NR 0 SEMS 0.500 0 0 0 NR NR 0 Federal CERCLIS NFRAP site list SEMS-ARCHIVE 0.500 0 0 0 NR NR 0 Federal RCRA CORRACTS facilities list CORRACTS 1.000 0 0 0 0 NR 0 Federal RCRA non-CORRACTS TSD facilities list RCRA-TSDF 0.500 0 0 0 NR NR 0 Federal RCRA generators list RCRA-LQG 0.250 0 0 NR NR NR 0 RCRA-SQG 0.250 0 0 NR NR NR 0 RCRA-CESQG 0.250 0 0 NR NR NR 0 Federal institutional controls / engineering controls registries LUCIS 0.500 0 0 0 NR NR 0 US ENG CONTROLS 0.500 0 0 0 NR NR 0 US INST CONTROL 0.500 0 0 0 NR NR 0 Federal ERNS list ERNS TP NR NR NR NR NR 0 State- and tribal - equivalent NPL HSL 1.000 0 0 0 0 NR 0 State- and tribal - equivalent CERCLIS CSCSL 1.000 0 0 0 0 NR 0 State and tribal landfill and/or solid waste disposal site lists SWF/LF 0.500 0 0 0 NR NR 0 State and tribal leaking storage tank lists LUST 0.500 0 0 0 NR NR -
U N S U U S E U R a C S
108th Congress of the United States C A N A D A CANADA Blaine Nooksack Trust Land Sumas Semiahmoo Bay 9 e Drayton t R Harbor t StRte 546 (Badger Rd) S Lynden Birch Bay Peaceful N o o Valley ks ac Nooksack Trust Land k R iv er Birch Bay Nooksack StRte 548 (Blaine Rd) Custer Everson Kendall Maple Falls StRte 548 (Grandview Rd) StRte 544 (Pole Rd) StRte 9 Strait of Georgia Glacier Nooksack Lake Terrell Trust Land Nooksack Trust Land ) y w Ferndale 2 H 4 r 5 e e k t a R t B Deming t S North Cascades Natl Pk n WHATCOM u o Ross (M Lake Nooksack Res StRte 542 Nooksack Trust Marietta- Land Alderwood OKANOGAN 5 Nooksack Trust Land Echo Bay Lummi Bellingham Res Boundary Pass President Channel Geneva Baker Lake Hales Passage StRte 9 (Valley Hwy) Sudden Hwy) es Bellingham cad Valley Acme as Bay 20 (C Ross Lake Natl Rec Area te R S t Cowlitz Bay Lake DISTRICT Chuckanut Whatcom Bay 5 New Channel Lake Samish S t West H w Alger y 1 Sound 1 Haro Strait ( C h East u S c tH k w Sound a y n Samish Bay u 2 Harney t 0 D Channel B ( San Juan Channel r) S e Lake Shannon t l R l i d n Samish TDSA 2 g 0 h ) Rosario Strait SAN JUAN a r m e Upright Upper v Rosario Strait i Channel C Skagit R h Edison a Res it Friday Lopez Sound n g nel a k Harbor S Friday Upper Skagit Res Hamilton Concrete North Cascades Natl Pk Lyman Harbor Guemes Channel StHwy 20 Ska Marblemount Sedro-Woolley git R iver Fidalgo Griffin Bay Bay Anacortes SKAGIT Rockport Padilla Bay View Bay Burrows Burlington Bay 0 StHwy 2 StH Clear Lake P w y Similk u (Memoria 5 StHwy 538 g l 36 Bay e H w t (College -

REGION 2 - Lower Mainland
REGION 2 - Lower Mainland CONTACT INFORMATION Fish and Wildlife Regional Office Salmon Information: (604) 586-4400 200-10428 153 St Fisheries and Oceans Canada Surrey BC V3R 1E1 District Offices (DFO) Conservation Officer Service Chilliwack: (604) 824-3300 Please call 1-877-952-7277 for recorded Delta: (604) 666-8266 information or to make an appointment at Langley: (604) 607-4150 any of the following Field Offices: Mission: (604) 814-1055 Mission, North Vancouver, Powell River, Squamish: (604) 892-3230 Sechelt, and Squamish Steveston: (604) 664-9250 Vancouver: (604) 666-0384 RAPP Shellfish Information line: (604) 666-2828 Report All Poachers Rand Polluters Mahood L i C in hilco Conservation Officerl 24 Hour Hotline tin k na STAY UP TO DATE: li R R 1-877-952-RAPPK (7277) iver ko Canim il Check website for in-season changes or h L Please refer to page 78 for more informationC closure dates for the 2021-2023 season rapp.bc.ca g at: www.gov.bc.ca/FishingRegulations r T o Cr a D C s y e 100 Mile House 5-6 e Tatlayoko k l o s o Lake M R r C 5-5 r 5-3 C CHILKO ig B Bonaparte n LAKE r L u R R h Taseko C te o ar hk Lakes ap at 5-4 3-31 on m FR B R Ho A S Y E a R n a R la k m o d m a R e Bish rd 3-32 D op o 2-15 L R R So Carpenter uthg ate ge Lake R Brid Gold ive Cache Creek Kamloops r Bridge R Lake 1-15 2-14 Seton BUTE L INLET 3-33 Anderson Lillooet 3-17 KAMLOOPS Phillips 2-13 L G R u i a R N Arm b r c o I O T C V h L h S o ILL s E OO o R P n E T o M y a O C C H r 2-11 3-16 T Sonora N TOBA ic Island R o INLET Pemberton la n E i e R l n a t e -

National Park Service U.S
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Exotic Plant Management Team Program 2009 Annual Report Natural Resource Report NPS/NRSS/NRR—2011/458 ON THE COVER Top center - Lake Mead EPMT, Curt Deuser; Center left - Gulf Coast EPMT working with a volunteer group from the National Wildlife Federation, Eric Worsham; Center center - Pacific Island EPMT using fire to treat invasive plants, Jeremy Gooding; Center right - Northern Rocky Mountains EPMT working in Bighorn Canyon NRA, Sue Salmons; Bottom left - Alaska EPMT using GPS to map occurrences of invasive plants, Bonnie Million; Bottom center - Alaska EPMT teaching children to identify invasive plants in Denali National Park, Bonnie Million ; Bottom right - Northern Great Plains EPMT treating leafy spurge at Devils Tower National Monument, Chad Prosser. Exotic Plant Management Team Program 2009 Annual Report Natural Resource Report NPS/NRSS/NRR—2011/458 Rita Beard National Park Service Invasive Plant Program Coordinator 1201 Oakridge Dr., Suite 200 Fort Collins, CO 80525 Louisa Gibson National Park Service Program Assistant 1201 Oakridge Dr., Suite 200 Fort Collins, CO 80525 October 2011 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. -

Biological Evaluation
BIOLOGICAL EVALUATION SKAGIT RIVER HYDROELECTRIC PROJECT LICENSE (FERC NO. 553) AMENDMENT: ADDITION OF A SECOND POWER TUNNEL AT THE GORGE DEVELOPMENT Final June 2011 BIOLOGICAL EVALUATION Table of Contents 1 INTRODUCTION .................................................................................................................. 1 1.1. Project Location .........................................................................................................2 1.2. Summary of Skagit River Project History ..................................................................2 1.2.1. Project Construction and Original License ................................................... 2 1.2.2. Settlement Agreement and Current License .................................................. 2 2 EXISTING PROJECT FACILITIES, OPERATIONS, AND MITIGATION AND CONSERVATION MEASURES ........................................................................................... 6 2.1. Existing Project Facilities ...........................................................................................6 2.1.1. Project Developments ................................................................................... 6 2.1.2. Diablo and Newhalem ................................................................................... 7 2.1.3. Project Transmission Lines ........................................................................... 7 2.2. Existing Operations – Reservoirs ...............................................................................8 2.2.1. Ross Lake -

September-2018.Pdf
NPS FY2018 FOIA LOG REGION FOIA NO. REQUESTED COMPLETED NAME ORGANIZATION SUBJECT POLICE REPORT #17‐079209 THAT OCCURRED AT APPROXIMATELY 5PM ON SEPTEMBER USPP NPS‐2018‐00002 09/28/17 11/17/17 SHADE 16, 2017. COPY OF MVA #17‐019856 INVOLVING [NAMED PARTY] THAT OCCURRED ON MARCH 9, USPP NPS‐2018‐00003 09/28/17 12/18/17 GONZALES 2017. COPY OF MVA #15‐086096 INVOLVING [NAMED PARTY] THAT OCCURRED ON SEPTEMBER USPP NPS‐2018‐00004 09/11/17 07/05/18 WINCHELL LAW OFFICE OF RICHARD R. KLEIN 15, 2017, ON SUITLAND PARKWAY AND NAYLOR ROAD. NERO NPS‐2018‐00005 09/29/17 01/16/18 DILLON NERO‐KATAHDIN WOODS‐ALL INFO 4/26/17 TO 8/31/17 COPY OF MV FATALITY #17‐037278 INVOLVING LUBA GIKHMAN THAT OCCURRED ON MAY USPP NPS‐2018‐00006 06/20/17 05/03/18 METROPOLITAN REPORTING METROPOLITAN REPORTING BUREAU 7, 2017, ON THE BALTIMORE WASHINGTON PARKWAY. USPP NPS‐2018‐00007 09/13/17 10/11/17 REIN THE WASHINGTON POST COPIES OF DOCUMENTS AS THEY RELATE TO REQUEST #NPS‐2017‐00940. PPFL; DSC; PRPA 15020 P17PS01286. SEEKING RECORDS REGARDING THE STEEL DSC NPS‐2018‐00008 09/26/17 10/24/17 KIDD ARAPAHO PIPE & SUPPLY LP FABRICATION SUPPLIER. COPY OF MVA #17‐13206 INVOLVING [NAMED PARTIES] THAT OCCURRED ON FEBRUARY USPP NPS‐2018‐00009 09/26/17 10/02/17 KAHN PROGRESSIVE GROUP OF INSURANCE CO15, 2017, ON THE BALTIMORE WASHINGTON PARKWAY. USPP NPS‐2018‐00010 09/26/17 ZEIGLER COPIES OF USPP GENERAL ORDERS SPEEDING TICKETS (NUMBER) ISSUED BY THE USPP ON THE GEORGE WASHINGTON PARKWAY SOUTH OF OLD TOWN ALEXANDRIA TO THE MOUNT VERNON CIRCLE DURING USPP NPS‐2018‐00011 09/26/17 10/30/17 CUNNINGHAM 2015. -

COLUMBIA Index, 1987-1996, Volumes 1
COLUMBIA The Magazine of Northwest History index 1987-1996 Volumes One through Ten Compiled by Robert C. Carriker and Mary E. Petty Published by the WashingtonState Historical Society with assistancefrom the WilliamL. DavisS.J Endowment of Gonzaga University Tacoma, Washington 1999 COLUMBIA The Magazine of Northwest History index 1987-1996 Volumes One through Ten EDITORS John McClelland, Jr., Interim Editor (1987-1988) and Founding Editor (1988-1996) David L. Nicandri, ExecutiveEditor (1988-1996) Christina Orange Dubois, AssistantEditor (1988-1991) and ManagingEditor/Desi gner (1992-1996) Robert C. Carriker, Book Review Editor ( 1987-1996) Arthur Dwelley, Associate Editor( 1988-1989) Cass Salzwedel, AssistantEditor (1987-1988) ArnyShepard Hines, Designer (1987-1991) Carolyn Simonson, CopyEditor ( 1991-1996) MANAGEMENT Christopher Lee, Business Manager (1988-1996) Gladys C. Para, CirculationManrtger (1987-1988) Marie De Long, Circulation Manager (1989-1996) EDITORIAL ADVISORS Knute 0. Berger (1987-1989) David M. Buerge (1987-1990) Keith A. Murray ( 1987-1989) J. William T. Youngs (1987-1991) Harold P. Simonson (1988-1989) Robert C. Wing (1989-1991) Arthur Dwelley (1990-1991) Robert A. Clark (1991) William L. Lang (1991-1992) STAFF CONTRIBUTORS Elaine Miller (1988-1996) JoyWerlink (1988-1996) Richard Frederick (1988-1996) Edward Nolan (1989-1996) Copyright © 1999 Washington State Historical Society All rights reserved. No part of this book may be reproduced in any form without permission fromthe publisher. ISBN 0-917048-72-5 Printed in the United States of America by Johnson-Cox Company INTRODUCTION COLUMBIA's initial index is the result of a two-year collaborative effort by a librarian and a historian. Standards established by professionals in the field were followed. -

Washington Seaplane Pilots Association North Cascades National Park – Ross Lake National Recreational Area Pilot Information B
Washington Seaplane Pilots Association North Cascades National Park – Ross Lake National Recreational Area Pilot Information Brief and Noise Abatement Procedure for Ross and Diablo Lakes This Pilot Information Brief/Noise Abatement Procedure is intended to provide guidelines for pilots flying to Ross or Diablo Lakes for day trips, overnight camping or while transiting on interstate travel. The North Cascades National Park Service is working in partnership with the Washington Seaplane Pilots’ Association (WSPA) to foster a cooperative relationship whereby seaplane pilots contribute to the Ross Lake NRA in a positive way, while working to minimize potential noise impact from their aircraft. Furthermore, WSPA provides training for its pilots related to this Noise Abatement Procedure. The fundamental objective of these procedures is to encourage pilots to proactively work with the National Parks Service to contribute to the quality of the park. This will be accomplished by taking steps to help maintain the quality of campsites, “leaving no trace”, and minimizing their impact on the park and its visitors, particularly with respect to noise. This document is primarily focused on Ross Lake as Diablo Lake is comparatively small with mountains on three sides and there are limited campsites with no available docks. Noise abatement procedures at Ross (and Diablo) Lakes are voluntary measures by pilots to “fly friendly” and be good neighbors to the other visitors. Pilots should only deviate from these procedures when necessary to comply with FAA regulations or in the interest of safety. These guidelines do not apply where it would conflict with Federal Aviation Regulations or where other procedures are considered necessary by a pilot to operate safely.