Nitric Oxide/Cyclic Guanosine Monophosphate Pathway in the Peripheral and Central Auditory System of the Rat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Biochemical Rationale for the Discrete Behavior of Nitroxyl and Nitric Oxide in the Cardiovascular System
A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system Katrina M. Miranda*†‡, Nazareno Paolocci§, Tatsuo Katori§, Douglas D. Thomas*, Eleonora Ford¶, Michael D. Bartbergerʈ, Michael G. Espey*, David A. Kass§, Martin Feelisch**, Jon M. Fukuto¶, and David A. Wink*† *Radiation Biology Branch, Building 10, Room B3-B69, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892; §Division of Cardiology, Department of Medicine, The Johns Hopkins Medical Institutions, Baltimore, MD 21287; ¶Department of Molecular and Medical Pharmacology, Center for the Health Sciences, University of California, Los Angeles, CA 90095; ʈDepartment of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095; and **Department of Molecular and Cellular Physiology, Louisiana State University Health Sciences Center, Shreveport, LA 71130 Edited by Louis J. Ignarro, University of California School of Medicine, Los Angeles, CA, and approved May 20, 2003 (received for review February 20, 2003) The redox siblings nitroxyl (HNO) and nitric oxide (NO) have often cellular thiol functions (14, 15). Conversely, NO reacts only indi- been assumed to undergo casual redox reactions in biological sys- rectly with thiols after RNOS formation (17). tems. However, several recent studies have demonstrated distinct Contrasting effects are also apparent in vivo or ex vivo,for pharmacological effects for donors of these two species. Here, infu- example in models of ischemia reperfusion injury. Exposure to NO sion of the HNO donor Angeli’s salt into normal dogs resulted in donors at the onset of reperfusion provides protection against elevated plasma levels of calcitonin gene-related peptide, whereas reperfusion injury in the heart and other organs (18–20). -

Fibroblast Growth Factor (Fgf) Implication in the Neonatal Development of the Cochlear Innervation G
FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION G. Després, I. Jalenques, R. Romand To cite this version: G. Després, I. Jalenques, R. Romand. FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION. Journal de Physique IV Proceedings, EDP Sciences, 1992, 02 (C1), pp.C1-173-C1-176. 10.1051/jp4:1992134. jpa-00251205 HAL Id: jpa-00251205 https://hal.archives-ouvertes.fr/jpa-00251205 Submitted on 1 Jan 1992 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C1, suppICment au Journal de Physique 111, Volume 2, avril 1992 FIBROBLAST GROWMI FACTOR (FGF) IMPLICATION IN TIlE NEONATAL DEVELOPMENT OF THE COCIiLEAR INNERVATION G. DESPR~S,I. JALENQUES and R. ROMAND Laboratoire de Neurobiolc@e et Physwlogie du Dkeloppement, Universite'BIaise Pascal, F-63177Aubi2re ceder, France The presence of fibroblast growth factor-like protein has been investigated on cryostat sections from Sprague Dawley rat cochleae and auditory brainstem nuclei of various neonatal stages by indirect immunofluorescence and immunoperoxydase techniques with an antibody directed against the 1-24 amino-acid sequence of brain derived basic FGF. -

5.1. Structure of the Spiral Ganglion
CHAPTER 5. INNERVATION OF THE ORGAN OF CORTI The investigation of nerve components of the acoustic system’s periph- eral part is so difficult methodologically that a whole series of questions which were solved long ago during the investigation of the other sensory system have not yet been clarified. The basic difficulty for the morphologists lies in the fact that the organ of Corti, together with its nerve elements, is located within the osseous tissue. In addition, it is in the shape of a spirally involut- ed geometrical figure. These structural peculiarities create considerable dif- ficulties during the determination of the connections between the types of peripheral and central neuron’s processes and bodies of the spiral ganglion of the cochlea. Therefore, most of the work on the cochlea’s innervation and the computation of the different element’s quantity demands an application of special methods, including graphical reconstruction of the serial sections. The Golgi method was and still remains the basic histological method of the organ of Corti’s innervation study, which has been supplemented by the cochlea’s electron-microscope investigations in the normal conditions and during the experimentally induced degenerations. 5.1. Structure of the spiral ganglion The neurons which innervate the auditory receptor cells form a spiral ganglion: a nerve-knot of the VIII pair’s acoustic part of the craniocerebral nerves. The ganglion fills the Rosental’s canal in the cochlea’s axis and re- peats the number of its spiral turns. The ganglionic neuron has, as a rule, a widened body with two processes: peripheral and central (Diagram 7). -

Auditory Neuropathy After Damage to Cochlear Spiral Ganglion Neurons
www.nature.com/scientificreports OPEN Auditory Neuropathy after Damage to Cochlear Spiral Ganglion Neurons in Mice Resulting from Conditional Received: 27 July 2016 Accepted: 15 June 2017 Expression of Diphtheria Toxin Published online: 25 July 2017 Receptors Haolai Pan1, Qiang Song1, Yanyan Huang1, Jiping Wang1, Renjie Chai2, Shankai Yin1 & Jian Wang 1,3 Auditory neuropathy (AN) is a hearing disorder characterized by normal cochlear amplifcation to sound but poor temporal processing and auditory perception in noisy backgrounds. These defcits likely result from impairments in auditory neural synchrony; such dyssynchrony of the neural responses has been linked to demyelination of auditory nerve fbers. However, no appropriate animal models are currently available that mimic this pathology. In this study, Cre-inducible diphtheria toxin receptor (iDTR+/+) mice were cross-mated with mice containing Cre (Bhlhb5-Cre+/−) specifc to spiral ganglion neurons (SGNs). In double-positive ofspring mice, the injection of diphtheria toxin (DT) led to a 30–40% rate of death for SGNs, but no hair cell damage. Demyelination types of pathologies were observed around the surviving SGNs and their fbers, many of which were distorted in shape. Correspondingly, a signifcant reduction in response synchrony to amplitude modulation was observed in this group of animals compared to the controls, which had a Cre− genotype. Taken together, our results suggest that SGN damage following the injection of DT in mice with Bhlhb5-Cre+/− and iDTR+/− is likely to be a good AN model of demyelination. Auditory neuropathy (AN) is a hearing disorder characterized as having normal cochlear microphonic (CM) potentials and otoacoustic emissions (OAEs), but largely reduced or missing auditory brainstem responses (ABRs). -

Download Product Insert (PDF)
Product Information DEA NONOate Item No. 82100 CAS Registry No.: 372965-00-9 Formal Name: Diethylammonium (Z)-1-(N,N- diethylamino)diazen-1-ium-1,2-diolate O Synonyms: DEA/NO, Diethylamine NONOate N MF: C4H10N3O2 • C4H12N + N N • H N FW: 206.3 2 Purity: ≥98% O- Stability: ≥1 year at -80°C Supplied as: A crystalline solid l UV/Vis.: max: 250 nm Laboratory Procedures For long term storage, keep DEA NONOate sealed under nitrogen at -80°C. It should be stable for at least one year. The crystals are sensitive to moisture and become discolored on exposure to air. Keep the vial sealed until use unless your laboratory is equipped with a glove box with an inert atmosphere for the handling of air sensitive compounds. DEA NONOate is supplied as a crystalline solid. A stock solution may be made by dissolving the DEA NONOate in an organic solvent purged with an inert gas. DEA NONOate is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF). The solubility of DEA NONOate is approximately 25 mg/ml in ethanol and 2 mg/ml in DMSO and DMF. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of DEA NONOate can be prepared by directly dissolving the crystalline compound in aqueous buffers. The solubility of DEA NONOate in PBS (pH 7.2) is approximately 10 mg/ml. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics
Review Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics Joel Mintz 1,†, Anastasia Vedenko 2,†, Omar Rosete 3 , Khushi Shah 4, Gabriella Goldstein 5 , Joshua M. Hare 2,6,7 , Ranjith Ramasamy 3,6,* and Himanshu Arora 2,3,6,* 1 Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Davie, FL 33328, USA; [email protected] 2 John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA; [email protected] (A.V.); [email protected] (J.M.H.) 3 Department of Urology, Miller School of Medicine, University of Miami, Miami, FL 33136, USA; [email protected] 4 College of Arts and Sciences, University of Miami, Miami, FL 33146, USA; [email protected] 5 College of Health Professions and Sciences, University of Central Florida, Orlando, FL 32816, USA; [email protected] 6 The Interdisciplinary Stem Cell Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, USA 7 Department of Medicine, Cardiology Division, Miller School of Medicine, University of Miami, Miami, FL 33136, USA * Correspondence: [email protected] (R.R.); [email protected] (H.A.) † These authors contributed equally to this work. Abstract: Nitric oxide (NO) is a short-lived, ubiquitous signaling molecule that affects numerous critical functions in the body. There are markedly conflicting findings in the literature regarding the bimodal effects of NO in carcinogenesis and tumor progression, which has important consequences for treatment. Several preclinical and clinical studies have suggested that both pro- and antitumori- Citation: Mintz, J.; Vedenko, A.; genic effects of NO depend on multiple aspects, including, but not limited to, tissue of generation, the Rosete, O.; Shah, K.; Goldstein, G.; level of production, the oxidative/reductive (redox) environment in which this radical is generated, Hare, J.M; Ramasamy, R.; Arora, H. -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -

Nitric Oxide Attenuates Hypoxia-Induced 5-FU Resistance
Sasabe et al. Int J Cancer Clin Res 2015, 2:2 DOI: 10.23937/2378-3419/2/2/1014 International Journal of Cancer and Clinical Research ISSN: 2378-3419 Original Article: Open Access Nitric Oxide Attenuates Hypoxia-Induced 5-FU Resistance of Oral Squamous Cell Carcinoma Cells Eri Sasabe*, Ayumi Tomomura, Mayuko Hamada, Naoya Kitamura, Tomohiro Yamada and Tetsuya Yamamoto Department of Oral and Maxillofacial Surgery, Kochi Medical School, Kochi University Kohasu, Japan *Corresponding author: Eri Sasabe, Department of Oral and Maxillofacial Surgery, Kochi Medical School, Kochi University Kohasu, Oko-cho, Nankoku, Kochi 783-8505, Japan, Tel: +81-88-880-2423, Fax: +81-88-880-2424, E-mail: [email protected] to development and progression of malignancies, and resistance Abstract to cancer therapy. The heterodimeric transcription factor Hypoxia Hypoxic environments in tumors induce expression of hypoxia- inducible factor (HIF) is a master regulator that can adapt tumor cells inducible factor (HIF). HIF contributes to the development of the to hypoxic conditions [1]. Overexpression of HIF has been observed malignant progression through the induction of various target genes. in many different cancers, including head and neck squamous cell We previously reported that treatment with chemotherapeutic drugs and γ-rays enhances expression and nuclear translocation carcinomas, and is associated with increased patient mortality [2-4]. of HIF-1α under normoxic conditions; susceptibility of oral HIF consists of an oxygen-regulated α-subunit and a constitutively squamous cell carcinoma (OSCC) cells to the drugs and γ-rays expressed β-subunit, also known as aryl hydrocarbon receptor is negatively correlated with expression of HIF-1α protein. -
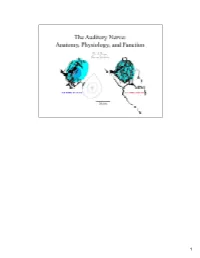
Auditory Nerve.Pdf
1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural signals representing these features are carried into the brain by the auditory nerve. It is thought that features of the sounds are processed centrally along parallel and hierarchical pathways where eventually percepts of the sounds are organized. 2 In mammals, the neural representation of acoustic information enters the brain by way of the auditory nerve. The auditory nerve terminates in the cochlear nucleus, and the cochlear nucleus in turn gives rise to multiple output projections that form separate but parallel limbs of the ascending auditory pathways. How the brain normally processes acoustic information will be heavily dependent upon the organization of auditory nerve input to the cochlear nucleus and on the nature of the different neural circuits that are established at this early stage. 3 This histology slide of a cat cochlea (right) illustrates the sensory receptors, the auditory nerve, and its target the cochlear nucleus. The orientation of the cut is illustrated by the pink line in the drawing of the cat head (left). We learned about the relationship between these structures by inserting a dye-filled micropipette into the auditory nerve and making small injections of the dye. After histological processing, stained single fibers were reconstruct back to their origin, and traced centrally to determine how they terminated in the brain. We will review the components of the nerve with respect to composition, innervation of the receptors, cell body morphology, myelination, and central terminations. -

Oxidative Stress and the Guanosine Nucleotide Triphosphate Pool: Implications for a Biomarker and Mechanism of Impaired Cell Function
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2008 OXIDATIVE STRESS AND THE GUANOSINE NUCLEOTIDE TRIPHOSPHATE POOL: IMPLICATIONS FOR A BIOMARKER AND MECHANISM OF IMPAIRED CELL FUNCTION Celeste Maree Bolin The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Bolin, Celeste Maree, "OXIDATIVE STRESS AND THE GUANOSINE NUCLEOTIDE TRIPHOSPHATE POOL: IMPLICATIONS FOR A BIOMARKER AND MECHANISM OF IMPAIRED CELL FUNCTION" (2008). Graduate Student Theses, Dissertations, & Professional Papers. 728. https://scholarworks.umt.edu/etd/728 This Dissertation is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. OXIDATIVE STRESS AND THE GUANOSINE NUCLEOTIDE TRIPHOSPHATE POOL: IMPLICATIONS FOR A BIOMARKER AND MECHANISM OF IMPAIRED CELL FUNCTION By Celeste Maree Bolin B.A. Chemistry, Whitman College, Walla Walla, WA 2001 Dissertation presented in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Toxicology The University of Montana Missoula, Montana Spring 2008 Approved by: Dr. David A. Strobel, Dean Graduate School Dr. Fernando Cardozo-Pelaez, -

Sustained Formation of Nitroglycerin-Derived Nitric Oxide
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2018/01/22/mol.117.110783.DC1 1521-0111/93/4/335–343$35.00 https://doi.org/10.1124/mol.117.110783 MOLECULAR PHARMACOLOGY Mol Pharmacol 93:335–343, April 2018 Copyright ª 2018 The Author(s). This is an open access article distributed under the CC BY Attribution 4.0 International license. Sustained Formation of Nitroglycerin-Derived Nitric Oxide by Aldehyde Dehydrogenase-2 in Vascular Smooth Muscle without Added Reductants: Implications for the Development of Nitrate Tolerance s Marissa Opelt, Gerald Wölkart, Emrah Eroglu, Markus Waldeck-Weiermair, Roland Malli, Wolfgang F. Graier, Alexander Kollau, John T. Fassett, Astrid Schrammel, Bernd Mayer, and Antonius C. F. Gorren Downloaded from Institute of Pharmaceutical Sciences, Department of Pharmacology and Toxicology, Karl-Franzens University (M.O., G.W., A.K., J.T.F., A.S., B.M., A.C.F.G.), and Institute of Molecular Biology and Biochemistry, Center of Molecular Medicine, Medical University Graz (E.E., M.W.-W., R.M., W.F.G.), Graz, Austria Received October 4, 2017; accepted January 18, 2018 molpharm.aspetjournals.org ABSTRACT According to current views, oxidation of aldehyde dehydrogenase-2 ALDH2, thiol-refractive inactivation was observed, particularly under (ALDH2) during glyceryltrinitrate (GTN) biotransformation is essen- high-turnover conditions. Organ bath experiments with rat aortas tially involved in vascular nitrate tolerance and explains the de- showed that relaxation by GTN lasted longer than that caused by the pendence of this reaction on added thiols. Using a novel fluorescent NO donor diethylamine/NONOate, in line with the long-lasting intracellular nitric oxide (NO) probe expressed in vascular smooth nanomolar NO generation from GTN observed in VSMCs.