Influence of Additives and Storage Temperature on Physicochemical and Microbiological Properties of Eye Drops Containing Cefazolin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aldrich Organometallic, Inorganic, Silanes, Boranes, and Deuterated Compounds
Aldrich Organometallic, Inorganic, Silanes, Boranes, and Deuterated Compounds Library Listing – 1,523 spectra Subset of Aldrich FT-IR Library related to organometallic, inorganic, boron and deueterium compounds. The Aldrich Material-Specific FT-IR Library collection represents a wide variety of the Aldrich Handbook of Fine Chemicals' most common chemicals divided by similar functional groups. These spectra were assembled from the Aldrich Collections of FT-IR Spectra Editions I or II, and the data has been carefully examined and processed by Thermo Fisher Scientific. Aldrich Organometallic, Inorganic, Silanes, Boranes, and Deuterated Compounds Index Compound Name Index Compound Name 1066 ((R)-(+)-2,2'- 1193 (1,2- BIS(DIPHENYLPHOSPHINO)-1,1'- BIS(DIPHENYLPHOSPHINO)ETHAN BINAPH)(1,5-CYCLOOCTADIENE) E)TUNGSTEN TETRACARBONYL, 1068 ((R)-(+)-2,2'- 97% BIS(DIPHENYLPHOSPHINO)-1,1'- 1062 (1,3- BINAPHTHYL)PALLADIUM(II) CH BIS(DIPHENYLPHOSPHINO)PROPA 1067 ((S)-(-)-2,2'- NE)DICHLORONICKEL(II) BIS(DIPHENYLPHOSPHINO)-1,1'- 598 (1,3-DIOXAN-2- BINAPH)(1,5-CYCLOOCTADIENE) YLETHYNYL)TRIMETHYLSILANE, 1140 (+)-(S)-1-((R)-2- 96% (DIPHENYLPHOSPHINO)FERROCE 1063 (1,4- NYL)ETHYL METHYL ETHER, 98 BIS(DIPHENYLPHOSPHINO)BUTAN 1146 (+)-(S)-N,N-DIMETHYL-1-((R)-1',2- E)(1,5- BIS(DI- CYCLOOCTADIENE)RHODIUM(I) PHENYLPHOSPHINO)FERROCENY TET L)E 951 (1,5-CYCLOOCTADIENE)(2,4- 1142 (+)-(S)-N,N-DIMETHYL-1-((R)-2- PENTANEDIONATO)RHODIUM(I), (DIPHENYLPHOSPHINO)FERROCE 99% NYL)ETHYLAMIN 1033 (1,5- 407 (+)-3',5'-O-(1,1,3,3- CYCLOOCTADIENE)BIS(METHYLD TETRAISOPROPYL-1,3- IPHENYLPHOSPHINE)IRIDIUM(I) -

Meeting Materials)
California State Board of Pharmacy BUSINESS, CONSUMER SERVICES AND HOUSING AGENCY 1625 N. Market Blvd, N219, Sacramento, CA 95834 DEPARTMENT OF CONSUMER AFFAIRS Phone: (916) 574-7900 GOVERNOR EDMUND G. BROWN JR. Fax: (916) 574-8618 www.pharmacy.ca.gov ENFORCEMENT AND COMPOUNDING COMMITTEE REPORT April 18, 2017 Amy Gutierrez, PharmD, Licensee Member, Chair Allen Schaad, Licensee Member, Vice Chair Greg Lippe, Public Member Stan Weisser, Licensee Member Valerie Muñoz, Public Member Ricardo Sanchez, Public Member I. Call to Order, Establishment of Quorum, and General Announcements II. Public Comments on Items Not on the Agenda/Agenda Items for Future Meetings Note: The board may not discuss or take action on any matter raised during this public comment section that is not included on this agenda, except to decide whether to place the matter on the agenda of a future meeting. [Government Code sections 11125, 11125.7(a)] III. Enforcement Matters a. University of California, San Diego’s Pilot Program to Permit Patients to Access Medications from an Automated Drug Delivery System Not Immediately Adjacent to the Pharmacy Background At the April 2015 Board Meeting, the board approved an 18-month pilot study under the auspices of the University of California, San Diego (UCSD) School of Pharmacy involving use of an automated drug delivery system (ADDS) for prescription medication from which staff of Sharp Hospital in San Diego and their families, who opted in, could pick up their outpatient medications. Consultation would be provided via telephone before medication could be dispensed to a patient for first time fills. Since that time the committee has received quarterly updates on the study, including usage of the system. -

Survey of Mercury and Mercury Compounds
Survey of mercury and mercury compounds Part of the LOUS-review Environmental Project No. 1544, 2014 Title: Authors and contributors: Survey of mercury and mercury compounds Jakob Maag Jesper Kjølholt Sonja Hagen Mikkelsen Christian Nyander Jeppesen Anna Juliana Clausen and Mie Ostenfeldt COWI A/S, Denmark Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. 2014 978-87-93026-98-8 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning research and development projects within the environmental sector, financed by study grants provided by the Danish Environmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. While the information provided in this report is believed to be accurate, the Danish Environmental Protection Agency disclaims any responsibility for possible inaccuracies or omissions and consequences that may flow from them. Neither the Danish Environmental Protection Agency nor COWI or any individual involved in the preparation of this publication shall be liable for any injury, loss, damage or prejudice of any kind that may be caused by persons who have acted based on their understanding of the information contained in this publication. Sources must be acknowledged. 2 Survey of mercury and mercury compounds Contents Preface ...................................................................................................................... 5 Summary and conclusions ......................................................................................... 7 Sammenfatning og konklusion ................................................................................ 14 1. -

WO 2018/069805 A2 19 April 2018 (19.04.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/069805 A2 19 April 2018 (19.04.2018) W !P O PCT (51) International Patent Classification: (84) Designated States (unless otherwise indicated, for every A61K 9/08 (2006.01) kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (21) International Application Number: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, PCT/IB20 17/056204 TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (22) International Filing Date: EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, 07 October 2017 (07.10.2017) MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, (25) Filing Language: English KM, ML, MR, NE, SN, TD, TG). (26) Publication Langi English Published: (30) Priority Data: — without international search report and to be republished 201621034602 10 October 2016 (10.10.2016) IN upon receipt of that report (Rule 48.2(g)) (71) Applicant: FTF PHARMA PRIVATE LIMITED [IN/IN]; Plot No. 183+23 1, Above Hyundai Service Centre, Navapura Char Rasta, NH 8A, Ahmedabad-Rajkot High way, Taluka-Sanand, Ahmedabad-382 210, Ahmedabad 382 210 (IN). (72) Inventors: PATEL, Vijay; B-40, Pulin Society Part 3,Near Gayatri School, BethakNaroda, Ahmedabad-382330 Gu jarat, Ahmedabad 382330 (IN). MEHTA, Sandip; D-74, New Jash Park Society, Isanpur, Ahmedabad-382443 Gu jarat, Ahmedabad 382443 (IN). -

PHENYLMERCURIC BORATE Phenylhydrargyri Boras PHENYLMERCURIC NITRATE Phenylhydrargyri Nitras
Phenylmercuric borate EUROPEAN PHARMACOPOEIA 7.0 Second identification: B, C. IDENTIFICATION A. Infrared absorption spectrophotometry (2.2.24). A. Infrared absorption spectrophotometry (2.2.24). Comparison: Ph. Eur. reference spectrum of Preparation:discs. phenylmercuric acetate. Comparison: Ph. Eur. reference spectrum of B. To 5 mL of solution S (see Tests) add 5 mL of water R and phenylmercuric borate. 0.1 mL of sodium sulfide solution R. A white precipitate is B. To 2 mL of solution S (see Tests) add 8 mL of water R and formed that darkens slowly on heating. 0.1 mL of sodium sulfide solution R. A white precipitate is C. To 10 mL of solution S add 2 mL of potassium iodide formed that darkens slowly on heating. solution R and shake vigorously. Filter. The filtrate gives C. Dissolve about 20 mg in 2 mL of methanol R.Thesolution reaction (b) of acetates (2.3.1). is clear and colourless. Ignite; the solution burns with a green-edged flame. TESTS Solution S. Dissolve 0.250 g in 40 mL of water R by heating to TESTS boiling. Allow to cool and dilute to 50 mL with water R. Prepare Solution S. Dissolve 0.25 g by sprinkling it on the surface of the solution immediately before use. 25 mL of boiling water R, cool and dilute to 25 mL with water R. Appearance of solution. Solution S is not more opalescent Appearance of solution. Solution S is clear (2.2.1)and than reference suspension II (2.2.1)andiscolourless(2.2.2, colourless (2.2.2, Method II). -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

PMB Technical Datasheet
PMB Technical Datasheet Product Information: Phenyl Mercuric Borate is a topical antiseptic and disinfectant that is soluble in water, ethanol and glycerol. It is used as Biocide in several products. Chemical Name PHENYLMERCURIC BORATE Grade Name PMB CAS No. 102-98-7 EINECS No. 203-068-1 Molecular Formula C6H5BHgO3 Synonyms MERPHEN FAMOSEPT SPIDOXOL TEST SPECIFICATION METHOD Solubility at Solvent Solubility ( gm/100ml ) Visual Product Applications: 1.It is used as an alternative antimicrobial preservative to phenylmercuric acetate or phenylmercuric nitrate. 2.It is used as an antiseptic. Product Benifits: 1.It is more soluble than phenylmercuric nitrate. 2.It is an antimicrobial preservative. Product Dosage: We strongly recommend testing of your own system under the actual conditions of processing and end-use prior to full scale testing. Exact loading must be determined by compositions of the specific polymer systems. Polymer Details Suggested dosage Product Handling & safety: Please refer to our product MSDS for specific instructions on handling this product. Product Disclaimer Important : This statement supersedes any Buyers documents. Seller makes no representation, Warranty, Express or Implied, Including of Merchantability of Fitness for a particular use, or purpose. No statement herein is to be construed as inducements to infringe any relevant patent. Under no circumstances shall Seler be liable for incidental, consequential or indirect damanges for alleges negligence breach of warranty, strict liability, and tort or contact rising in connectoin with product(s). Buyers sole remedy and Sellers sole Liability for any claims shall be buyers purchase price. Data and results are based on controlled or lab work and must be confirmed by the buyer by testing for its indented conditions of use. -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated July 30, 2020 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Notice of Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List • Additions to category 1: Betahistine Hydrochloride L-Proline Citrulline Papaverine Hydrochloride* Copper Gluconate Phosphatidylcholine Diiodohydroxyquinoline Podophyllum Resin Glucosamine Sulfate Potassium Chloride Sodium Molybdate Glucosamine Sulfate Sodium Chloride Sodium Nitroprusside Hydrochloric Acid* Sodium Selenite/Sodium Selenite Pentahydrate* Ibutamoren Mesylate Trichloroacetic Acid* L-Citrulline* Ubidecarenone (Coenzyme Q10)* L-Lysine * Bulk drug substance is currently in Category 3 and is being moved to Category 1. • Minor revision to entry in Category 1 to correct a misspelling: o Correct the misspelling of “cimatidine” to “cimetidine” 1 Updated July 30, 2020 503B Category 1: Bulk Drug Substances Under Evaluation • 5-Methyltetrahydrofolate Calcium • Chromic Chloride • 17-alpha-Hydroxyprogesterone • Chromium chloride/ Chromium Chloride • Acetylcysteine Hexahydrate • Acyclovir • Ciclopirox Oleate • Adapalene • Cimetidine • Adenosine • Ciprofloxacin HCl • Allantoin • Citric Acid Anhydrous • Alpha Lipoic Acid • Citrulline/L-Citrulline • Alprostadil • Clindamycin Phosphate -

Antimicrobial Activity of Seven Metallic Compounds Against Penicillinase Producing and Non-Penicillinase Producing Strains of Neisseria Gonorrhoeae
Genitourin Med: first published as 10.1136/sti.62.3.163 on 1 June 1986. Downloaded from Genitourin Med 1986;62:163-5 Antimicrobial activity of seven metallic compounds against penicillinase producing and non-penicillinase producing strains of Neisseria gonorrhoeae M PEETERS,* D VANDEN BERGHE,* AND A MEHEUSt From the Departments of *Microbiology and tEpidemiology and Social Medicine, University ofAntwerp, Antwerp, Belgium SUMMARY The in vitro activity of seven metallic compounds was tested against penicillinase (B lactamase) producing strains of Neisseria gonorrhoeae (PPNG) and non-PPNG strains. On a weight basis, the mercurials showed the greatest in vitro activity. Phenylmercuric borate, thiomersal, and mercuric chloride inhibited 90% of all strains at concentrations of 5 mg/l, 5 mg/l, and 20 mgA respectively. Silver nitrate inhibited 90% ofthe strains at 80 mg/l and the MIC90 for mild silver protein was 200 mg/l. Copper and selenium salts had lower in vitro activities, inhibiting 90% of all the strains at 320 mg/l and 640 mgA respectively. Silver nitrate and the six other compounds tested showed equal activities against PPNG and non-PPNG strains. This finding supports the recommendation for prophylaxis of gonococcal conjunctivitis ofthe newborn with 1% silver nitrate eye drops. Introduction gonococcal ophthalmia caused by PPNG strains. In this study, we tested the in vitro activity of silver Gonococcal ophthalmia neonatorum was a leading nitrate and six other antimicrobial metallic com- cause ofchildhood blindness before the penicillin era.' pounds against PPNG and non-PPNG strains. Prevention was essential. In 1881 Crede introduced http://sti.bmj.com/ silver nitrate for prophylaxis, and its use became Materials and methods widespread. -
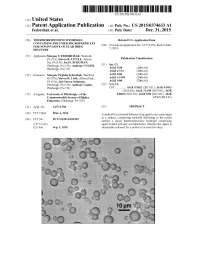
(12) Patent Application Publication (10) Pub. No.: US 2015/0374633 A1 Fedorchak Et Al
US 20150374633A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0374633 A1 Fedorchak et al. (43) Pub. Date: Dec. 31, 2015 (54) THERMORESPONSIVE HYDROGEL Related U.S. Application Data CONTAINING POLYMERMICROPARTICLES FOR NONINVASIVE OCULAR DRUG (60) Eyinal application No. 61/773,076, filed on Mar. DELIVERY s (71) Applicants: Morgan V. FEDORCHAK, Wexford, O O PA (US); Steven R. LITTLE, Allison Publication Classification Par, PA (US); Joel S. SCHUMAN, E.- EA SS Anthony CUGINI, (51) A6IK9/50Int. Cl. (2006.01) s A6IK47/34 (2006.01) (72) Inventors: Morgan Virginia Fedorchak, Wexford, A6IX 9/06 (2006.01) PA (US); Steven R. Little, Allison Park, A6IX3 L/498 (2006.01) PA (US); Joel Steven Schuman, A6IK9/00 (2006.01) Pittsburgh, PA (US); Anthony Cugini, (52) U.S. Cl. Pittsburgh, PA (US) CPC ............. A61K 9/5021 (2013.01); A61 K9/5031 (2013.01); A61 K3I/498 (2013.01); A61 K (73) Assignee: University of Pittsburgh - of the 9/0048 (2013.01); A61K 9/06 (2013.01); A6IK Commonwealth System of Higher 47/34 (2013.01) Education, Pittsburgh, PA (US) (21) Appl. No.: 14/772,758 (57) ABSTRACT (22) PCT Filed: Mar. 4, 2014 A method for Sustained delivery of an agent to an ocular organ in a subject, comprising topically delivering to the ocular (86). PCT No.: PCT/US2014/020355 Surface a liquid thermoresponsive hydrogel comprising S371 (c)(1), agent-loaded polymer microparticles, wherein the agent is (2) Date: Sep. 3, 2015 sustainably released for a period of at least five days. Patent Application Publication Dec. 31, 2015 Sheet 1 of 9 US 2015/0374633 A1 F.G. -
Gpi Drug Classification Report
GPI CLASSIFICATION REPORT NAME CODE Digit Match Level Count ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS 6100000000 2 digit 1 ALTERNATIVE MEDICINES 9500000000 2 digit 2 AMEBICIDES 1400000000 2 digit 3 AMINOGLYCOSIDES 0700000000 2 digit 4 ANALGESICS - ANTI-INFLAMMATORY 6600000000 2 digit 5 ANALGESICS - NONNARCOTIC 6400000000 2 digit 6 ANALGESICS - OPIOID 6500000000 2 digit 7 ANDROGENS-ANABOLIC 2300000000 2 digit 8 ANORECTAL AGENTS 8900000000 2 digit 9 ANTACIDS 4800000000 2 digit 10 ANTHELMINTICS 1500000000 2 digit 11 ANTI-INFECTIVE AGENTS - MISC. 1600000000 2 digit 12 ANTIANGINAL AGENTS 3200000000 2 digit 13 ANTIANXIETY AGENTS 5700000000 2 digit 14 ANTIARRHYTHMICS 3500000000 2 digit 15 ANTIASTHMATIC AND BRONCHODILATOR AGENTS 4400000000 2 digit 16 ANTICOAGULANTS 8300000000 2 digit 17 ANTICONVULSANTS 7200000000 2 digit 18 ANTIDEPRESSANTS 5800000000 2 digit 19 ANTIDIABETICS 2700000000 2 digit 20 ANTIDIARRHEALS 4700000000 2 digit 21 ANTIDOTES 9300000000 2 digit 22 ANTIEMETICS 5000000000 2 digit 23 ANTIFUNGALS 1100000000 2 digit 24 ANTIHISTAMINES 4100000000 2 digit 25 ANTIHYPERLIPIDEMICS 3900000000 2 digit 26 ANTIHYPERTENSIVES 3600000000 2 digit 27 ANTIMALARIALS 1300000000 2 digit 28 LEGEND: 2 Digit = MAJOR CLASS - MATCH OFF OF FIRST TWO DIGITS OF GPI 4 Digit = MAJOR SUB CLASS - MATCH OFF OF FIRST FOUR DIGITS OF GPI 6 Digit = MINOR SUB CLASS - MATCH OFF OF FIRST SIX DIGITS OF GPI Printed:11/13/2012 10 Digit = MEDICATION NAME - MATCH OFF OF ALL TEN DIGITS OF GPI Lee Cooper Page 1 of 265 NAME CODE Digit Match Level Count ANTIMYASTHENIC AGENTS