2014 US National List of Reportable Animal Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Much Do You Know About Fleas, Ticks, Mites and Other Biters? by Vet Glen Cousquer C Norris
Artful arthropods How much do you know about fleas, ticks, mites and other biters? By vet Glen Cousquer C Norris robably the most historically significant and well known arthropod-borne disease was the “Black Death”. This scourge of the Middle Ages, also known as the Bubonic Plague, killed Pbetween 30 and 60 per cent of Europe’s human population during the 14th Century. This bacterial disease was carried by black rats (Rattus rattus) and transmitted from the rat to humans by the Oriental rat flea (Xenopsylla cheopis). The flea was able to pick up the disease when feeding on rats and then transfer Cheyletiella mites result in the appearance the disease when feeding again on humans. At the time, the of scurf and bald patches in the fur various contributing causes of this devastating disease remained G Cousquer obscure. Our ancestors were largely ignorant of the role played transmit diseases to rabbits. Many of the most important rabbit by the rat, the flea, poor hygiene and inadequate sanitation and diseases are transmitted in this way and we need a detailed this severely hampered their attempts to deal with outbreaks. understanding of the process involved if we are to protect our Today, it is this understanding of how disease can be carried rabbits’ health and welfare. by reservoirs and then transmitted by vectors that allows us to mount more effective responses to such disease threats. So, what are the common arthropod parasites found feeding on rabbits? This feature will look at how certain arthropods play key roles in the transmission of diseases to rabbits. -

TICKS in RELATION to HUMAN DISEASES CAUSED by <I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 1967 TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES Harry Hoogstraal Follow this and additional works at: https://digitalcommons.unl.edu/usnavyresearch This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in U.S. Navy Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES1,2 By HARRY HOOGSTRAAL Department oj Medical Zoology, United States Naval Medical Research Unit Number Three, Cairo, Egypt, U.A.R. Rickettsiae (185) are obligate intracellular parasites that multiply by binary fission in the cells of both vertebrate and invertebrate hosts. They are pleomorphic coccobacillary bodies with complex cell walls containing muramic acid, and internal structures composed of ribonucleic and deoxyri bonucleic acids. Rickettsiae show independent metabolic activity with amino acids and intermediate carbohydrates as substrates, and are very susceptible to tetracyclines as well as to other antibiotics. They may be considered as fastidious bacteria whose major unique character is their obligate intracellu lar life, although there is at least one exception to this. In appearance, they range from coccoid forms 0.3 J.I. in diameter to long chains of bacillary forms. They are thus intermediate in size between most bacteria and filterable viruses, and form the family Rickettsiaceae Pinkerton. They stain poorly by Gram's method but well by the procedures of Macchiavello, Gimenez, and Giemsa. -

WHAT IS MYXOMATOSIS? After Its Introduction to the UK in the Early 1950’S Myxomatosis Was So Virulent That an Estimated 95% of Wild and Pet Rabbits Had Died by 1955
WHAT IS MYXOMATOSIS? After its introduction to the UK in the early 1950’s myxomatosis was so virulent that an estimated 95% of wild and pet rabbits had died by 1955. In the year 2000 we again experienced one of the worst outbreaks of Myxomatosis ever recorded, and this disease is still affecting rabbits, particularly during the late summer/ autumn season. Below: edited extracts from an article written by Dr Linda Dykes, published in Fur & Feather’s November 2000 issue. yxomatosis is a rabbit will be a pitiful sight. Severe viral disease which conjunctivitis causes blindness and decimated the wild is accompanied by swelling of the Mrabbit population when it head and genital region plus lumps arrived in Britain nearly seventy on the body. years ago. The number and The rabbit can take a fortnight to severity of outbreaks varies over die and treatment of the “classic” time as the myxomatosis virus form of myxomatosis is usually is notorious for its ability to futile. mutate from year to year, and the background immunity in There are also two atypical forms the wild rabbit population also of myxomatosis: one causes varies. pneumonia and a snuffles-like illness; the other (“nodular Be especially careful if you have a being a bit “off colour” with a few Rabbits at myxomatosis”) mainly affects skin Greatest Risk dog or cat that hunts wild rabbits, skin lesions. and carries a better prognosis. Domestic rabbits do not have as they could bring rabbit fleas Treatment is usually successful any genetically based immunity If a vaccinated rabbit does develop home or into the rabbitry on their in the vaccinated rabbit with a against myxomatosis. -

Wildlife Disease Monitor 2014
WILDLIFE DISEASE MONITORING IN SWEDEN 2014 Editors: Jonas Malmsten, Erik Ågren Authors: Caroline Bröjer, Gete Hestvik, Aleksija Neimanis, Jonas Malmsten, Torsten Mörner, Henrik Uhlhorn, Erik Ågren Photo, front page: Jim Hallander Layout: Gun-Britt Rydén, Jonas Malmsten Suggested citation: Wildlife disease monitoring in Sweden 2014. National Veterinary Institute, SVA, Uppsala, Sweden. SVA´s report series 33, ISSN 1654-7098 Address: Ulls väg 2 B, 751 89 Uppsala telephone. +46 18 67 40 00 fax. +46 18 30 91 62 e-mail. [email protected] webb. www.sva.se Table of contents Foreword ....................................................... 1 Wild boar as carriers of pathogenic Wildlife disease surveillance in Sweden ......... 2 agents ..................................................... 13 Staff working with Wildlife disease Bird flu monitoring ................................... 13 investigations in 2014 ................................ 2 Investigation of moose calf mortality on Wildlife diseases and increased mortality Öland ...................................................... 14 events of interest 2014 .................................. 3 Pasteurellosis in fallow deer .................... 14 Bird flu virus in harbour seals .................... 3 PCR diagnosis of trichomonas infection in Fox tapeworm – EchinococcosIS .............. 3 wild birds ................................................. 14 Mortality event in fallow deer ..................... 3 Salmonella in hedgehogs ........................ 15 Trichomoniasis in Greenfinches ............... -

Arthropod Parasites in Domestic Animals
ARTHROPOD PARASITES IN DOMESTIC ANIMALS Abbreviations KINGDOM PHYLUM CLASS ORDER CODE Metazoa Arthropoda Insecta Siphonaptera INS:Sip Mallophaga INS:Mal Anoplura INS:Ano Diptera INS:Dip Arachnida Ixodida ARA:Ixo Mesostigmata ARA:Mes Prostigmata ARA:Pro Astigmata ARA:Ast Crustacea Pentastomata CRU:Pen References Ashford, R.W. & Crewe, W. 2003. The parasites of Homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Taylor & Francis. Taylor, M.A., Coop, R.L. & Wall, R.L. 2007. Veterinary Parasitology. 3rd edition, Blackwell Pub. HOST-PARASITE CHECKLIST Class: MAMMALIA [mammals] Subclass: EUTHERIA [placental mammals] Order: PRIMATES [prosimians and simians] Suborder: SIMIAE [monkeys, apes, man] Family: HOMINIDAE [man] Homo sapiens Linnaeus, 1758 [man] ARA:Ast Sarcoptes bovis, ectoparasite (‘milker’s itch’)(mange mite) ARA:Ast Sarcoptes equi, ectoparasite (‘cavalryman’s itch’)(mange mite) ARA:Ast Sarcoptes scabiei, skin (mange mite) ARA:Ixo Ixodes cornuatus, ectoparasite (scrub tick) ARA:Ixo Ixodes holocyclus, ectoparasite (scrub tick, paralysis tick) ARA:Ixo Ornithodoros gurneyi, ectoparasite (kangaroo tick) ARA:Pro Cheyletiella blakei, ectoparasite (mite) ARA:Pro Cheyletiella parasitivorax, ectoparasite (rabbit fur mite) ARA:Pro Demodex brevis, sebacceous glands (mange mite) ARA:Pro Demodex folliculorum, hair follicles (mange mite) ARA:Pro Trombicula sarcina, ectoparasite (black soil itch mite) INS:Ano Pediculus capitis, ectoparasite (head louse) INS:Ano Pediculus humanus, ectoparasite (body -

CDFA List of Reportable Conditions for Animals and Animal Products
January 2021 ANIMAL HEALTH BRANCH LIST OF REPORTABLE CONDITIONS FOR ANIMALS AND ANIMAL PRODUCTS* *Pursuant to Section 9101 of the California Food and Agricultural Code, Title 3 California Code of Regulations § 797 and Title 9 Code of Federal Regulations Section 161.4(f) WHO MUST REPORT: Any licensed veterinarian, any person operating a diagnostic laboratory, or any person who has been informed, recognizes or should recognize by virtue of education, experience, or occupation, that any animal or animal product is or may be affected by, or has been exposed to, or may be transmitting or carrying any of the following conditions, must report that information. WHAT TO REPORT: Immediately report any animal disease not known to exist in the United States, any event with increased mortality and/or morbidity of unknown cause or source and any toxicology condition likely to contaminate animals or animal products (meat, milk or eggs). CALL IF YOU SEE: high morbidity or mortality, vesicles, CNS signs, uncommon ticks, hemorrhagic septicemias, unusual larvae in wounds, unusual or unexplained illness. Report any emergency, regulatory, or monitored condition within the provided time frame. Some diseases are listed under the major species of concern; if you see compatible signs for such conditions in another species, please report! EMERGENCY CONDITIONS REGULATORY CONDITIONS MONITORED CONDITIONS Report within 24 Hours of Discovery Report within Two Days of Discovery Report within 30 Days of Discovery MULTIPLE SPECIES MULTIPLE SPECIES MULTIPLE SPECIES • Brucellosis (B. melitensis, B. abortus, B. suis)1 • Bluetongue General, non-specific conditions: Unexplained high • Pseudorabies (Aujeszky’s disease) • Echinococcosis/hydatidosis (Echinococcus species) mortality or diseased animals; livestock exposed to toxic • Tuberculosis (Mycobacterium bovis)1 • Epizootic hemorrhagic disease substances. -

Wildlife Parasitology in Australia: Past, Present and Future
CSIRO PUBLISHING Australian Journal of Zoology, 2018, 66, 286–305 Review https://doi.org/10.1071/ZO19017 Wildlife parasitology in Australia: past, present and future David M. Spratt A,C and Ian Beveridge B AAustralian National Wildlife Collection, National Research Collections Australia, CSIRO, GPO Box 1700, Canberra, ACT 2601, Australia. BVeterinary Clinical Centre, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Werribee, Vic. 3030, Australia. CCorresponding author. Email: [email protected] Abstract. Wildlife parasitology is a highly diverse area of research encompassing many fields including taxonomy, ecology, pathology and epidemiology, and with participants from extremely disparate scientific fields. In addition, the organisms studied are highly dissimilar, ranging from platyhelminths, nematodes and acanthocephalans to insects, arachnids, crustaceans and protists. This review of the parasites of wildlife in Australia highlights the advances made to date, focussing on the work, interests and major findings of researchers over the years and identifies current significant gaps that exist in our understanding. The review is divided into three sections covering protist, helminth and arthropod parasites. The challenge to document the diversity of parasites in Australia continues at a traditional level but the advent of molecular methods has heightened the significance of this issue. Modern methods are providing an avenue for major advances in documenting and restructuring the phylogeny of protistan parasites in particular, while facilitating the recognition of species complexes in helminth taxa previously defined by traditional morphological methods. The life cycles, ecology and general biology of most parasites of wildlife in Australia are extremely poorly understood. While the phylogenetic origins of the Australian vertebrate fauna are complex, so too are the likely origins of their parasites, which do not necessarily mirror those of their hosts. -
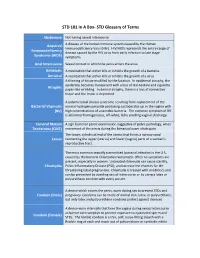
STD Glossary of Terms
STD 101 In A Box- STD Glossary of Terms Abstinence Not having sexual intercourse Acquired A disease of the human immune system caused by the Human Immunodeficiency Virus (HIV). HIV/AIDS represents the entire range of Immunodeficiency disease caused by the HIV virus from early infection to late stage Syndrome (AIDS) symptoms. Anal Intercourse Sexual contact in which the penis enters the anus. Antibiotic A medication that either kills or inhibits the growth of a bacteria. Antiviral A medication that either kills or inhibits the growth of a virus. A thinning of tissue modified by the location. In epidermal atrophy, the epidermis becomes transparent with a loss of skin texture and cigarette Atrophic paper-like wrinkling. In dermal atrophy, there is a loss of connective tissue and the lesion is depressed. A polymicrobial clinical syndrome resulting from replacement of the Bacterial Vaginosis normal hydrogen peroxide producing Lactobacillus sp. in the vagina with (BV) high concentrations of anaerobic bacteria. The common symptom of BV is abnormal homogeneous, off-white, fishy smelling vaginal discharge. Cervical Motion A sign found on pelvic examination suggestive of pelvic pathology; when Tenderness (CMT) movement of the cervix during the bimanual exam elicits pain. The lower, cylindrical end of the uterus that forms a narrow canal Cervix connecting the upper (uterus) and lower (vagina) parts of a woman's reproductive tract. The most common sexually transmitted bacterial infection in the U.S., caused by the bacteria Chlamydia trachomatis. Often no symptoms are present, especially in women. Untreated chlamydia can cause sterility, Chlamydia Pelvic Inflammatory Disease (PID), and increase the chances for life- threatening tubal pregnancies. -

The Evolution of Flea-Borne Transmission in Yersinia Pestis
Curr. Issues Mol. Biol. 7: 197–212. Online journal at www.cimb.org The Evolution of Flea-borne Transmission in Yersinia pestis B. Joseph Hinnebusch al., 1999; Hinchcliffe et al., 2003; Chain et al., 2004). Presumably, the change from the food- and water-borne Laboratory of Human Bacterial Pathogenesis, Rocky transmission of the Y. pseudotuberculosis ancestor to Mountain Laboratories, National Institute of Allergy the flea-borne transmission of Y. pestis occurred during and Infectious Diseases, National Institutes of Health, this evolutionarily short period of time. The monophyletic Hamilton, MT 59840 USA relationship of these two sister-species implies that the genetic changes that underlie the ability of Y. pestis to use Abstract the flea for its transmission vector are relatively few and Transmission by fleabite is a recent evolutionary adaptation discrete. Therefore, the Y. pseudotuberculosis –Y. pestis that distinguishes Yersinia pestis, the agent of plague, species complex provides an interesting case study in from Yersinia pseudotuberculosis and all other enteric the evolution of arthropod-borne transmission. Some of bacteria. The very close genetic relationship between Y. the genetic changes that led to flea-borne transmission pestis and Y. pseudotuberculosis indicates that just a few have been identified using the rat flea Xenopsylla cheopis discrete genetic changes were sufficient to give rise to flea- as model organism, and an evolutionary pathway can borne transmission. Y. pestis exhibits a distinct infection now be surmised. Reliance on the flea for transmission phenotype in its flea vector, and a transmissible infection also imposed new selective pressures on Y. pestis that depends on genes that are specifically required in the help explain the evolution of increased virulence in this flea, but not the mammal. -

Common Rabbit Pathogens
Common Rabbit Pathogens Encephalitozoon Cuniculi Encephalitozoonosis is caused by Encephalitozoon cuniculi. About 80% of healthy rabbits carry the pathogen, without any clinical signs developing. Clinical disease can present with the following clinical signs: torticollis, ataxia, uveitis, posterior paresis and urinary incontinence. Other clinical signs can occur depending on the particular organs involved (such as hepatic and renal disease). Transmission is by infectious spores excreted primarily in the urine but also in the faeces and transmission can occur both orally and nasally. A pregnant doe can pass the pathogen on to her offspring in utero. The disease is a zoonosis and is an emerging human pathogen. Detection is now available by two methods both the traditional serology, giving antibody titres, to enable monitoring of clinical cases or detection of active shedding by PCR. The options available to help diagnose E. cuniculi are: Serology An IgG titre is the most commonly used test, this indicates long term exposure. Levels continue to rise steadily from 30 days post primary infection until they peak at 70 days. An IgM titre is now also available and can be used in conjunction with the IgG. IgM indicates early exposure and new infection in the initial 35 days prior to IgG detection. The sample required is serum (not from a gel tube). PCR No special medium is required for this test, a sample of urine, CSF or faeces simply needs to be placed in to a sterile universal tube. The sensitivity of this test is 96%, the specificity has yet to be measured. E. cuniculi can be intermittently shed, so a 3 day pooled sample should be taken. -

BIO 475 - Parasitology Spring 2009 Stephen M
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 24 Subphylum Hexapoda (Insecta) 1.Characteristics a. Six legs, b. Head, thorax abdomen c. Often with winged adults 2. Main Parasitic Orders a. Mallophaga b. Anoplura c. Hemiptera d. Siphonaptera e. Diptera 1 Order Mallophaga Order Anoplura Order Anoplura 1.Vectors of disease a. Rickettsia (typhus) b. Rhochalimaea (trench fever) c. Borrelia (relapsing fever) 2. Important species a. Pediculus humanus humanus (clothing) b. Pediculus humanus capitus (smaller, head) c. Phthirius pubis 2 3 4 Phthirius pubis Infestation 5 6 Order Siphonaptera 1. Are holometabolous insects. a. Have egg -> larvae-> pupae - > adult. Order Siphonaptera Excellent jumpers: Click mechanism in thorax permits 140g force in 1/1000 sec. Equals 800 foot standing jump! Order Siphonaptera Often host specific with particular affinities. Associated with nests or hosts, hair or feathers, surface or burrowing. 7 Nest Associated Most rodent fleas. Including prairie dogs. Xenopsylla cheopis Disease Transmission Plague (Yersinia pestis). Usually associated with Xenopsylla cheopis. Also: Murine typhus Myxomatosis Plague Transmission 8 The bubonic plague was the most commonly seen form of the Black Death. The mortality rate was 30- 75%. The symptoms were enlarged and inflamed lymph nodes (around arm pits, neck and groin). The term 'bubonic' refers to the characteristic bubo or enlarged lymphatic gland. Victims were subject to headaches, nausea, aching joints, fever of 101-105 degrees, vomiting, and a general feeling of illness. Symptoms took from 1-7 days to appear. 9 Host Associated Most fleas including: The human flea, Pulex irritans Rabbit fleas, Nosopsyllus Cat fleas, Ctenocephalides Attached Fleas Chicken fleas (stick tight fleas) Echidophaga Burrowing Fleas Tunga penetrans 10 11 12 13 14 15. -

Understanding Myxomatosis
CH •X ANG DF E P Unit T, Viking House, Daneholes Roundabout, Grays, Essex, RM16 2XE, w Click to buy NOW! Tel: 01375 399033, Fax: 01375 385129, Web: www.allanimalsvetclinic.co.uk, w m o w c .d k. ocu•trac Email: [email protected] RABBIT FACT SHEET 1. Understanding Myxomatosis What is myxomatosis? Myxomatosis is a severe viral disease of rabbits that decimated the wild rabbit population when it arrived in Britain 50 years ago. Domestic rabbits are also susceptible to the disease and deaths in pets are reported every year. Is my rabbit at risk? Myxomatosis poses a threat to all pet rabbits – but the risk varies depending on whether your rabbit lives inside or outside. Pet rabbits at greatest risk are those living outside, especially if they may have any contact with wild rabbits or hares. Pet rabbits affected by rabbit fleas are also at very high risk • rabbit owners who also have a dog or cat that hunts wild rabbits (or foxes that visit the garden and nose around rabbit hutches) must be particularly careful, in case rabbit fleas are brought back to the pet bunny. Houserabbits living permanently indoors are at less risk than outdoor rabbits, but can and do get myxomatosis. They must be vaccinated and protected from possible sources of myxomatosis transmission too. How is it spread? Pet rabbits could contract myxomatosis in a variety of ways: • Bites from mosquitoes carrying the Myxoma virus. • Bites from fleas carrying the Myxoma virus (fleas can survive for many months in hay). • Myxomatosis can also be spread by Cheyletiella fur mites.