Lineage‐Specific Plastid Degradation in Subtribe Gentianinae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
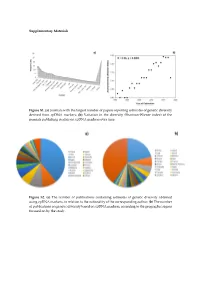
(A) Journals with the Largest Number of Papers Reporting Estimates Of
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

A Review of European Progress Towards the Global Strategy for Plant Conservation 2011-2020
A review of European progress towards the Global Strategy for Plant Conservation 2011-2020 1 A review of European progress towards the Global Strategy for Plant Conservation 2011-2020 The geographical area of ‘Europe’ includes the forty seven countries of the Council of Europe and Belarus: Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia-Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Republic of Moldova, Monaco, Montenegro, Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, Russian Federation, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom. Front Cover Image: Species rich meadow with Papaver paucifoliatum, Armenia, Anna Asatryan. Disclaimer: The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the copyright holders concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mentioning of specific companies or products does not imply that they are endorsed or recommended by PLANTA EUROPA or Plantlife International or preferred to others that are not mentioned – they are simply included as examples. All reasonable precautions have been taken by PLANTA EUROPA and Plantlife International to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall PLANTA EUROPA, Plantlife International or the authors be liable for any consequences whatsoever arising from its use. -

Samenkatalog Graz 2016.Pdf
SAMENTAUSCHVERZEICHNIS Index Seminum Seed list Catalogue de graines des Botanischen Gartens der Karl-Franzens-Universität Graz Ernte / Harvest / Récolte 2016 Herausgegeben von Christian BERG, Kurt MARQUART & Jonathan WILFLING ebgconsortiumindexseminum2012 Institut für Pflanzenwissenschaften, Januar 2017 Botanical Garden, Institute of Plant Sciences, Karl- Franzens-Universität Graz 2 Botanischer Garten Institut für Pflanzenwissenschaften Karl-Franzens-Universität Graz Holteigasse 6 A - 8010 Graz, Austria Fax: ++43-316-380-9883 Email- und Telefonkontakt: [email protected], Tel.: ++43-316-380-5651 [email protected], Tel.: ++43-316-380-5747 Webseite: http://garten.uni-graz.at/ Zitiervorschlag : BERG, C., MARQUART, K. & Wilfling, J. (2017): Samentauschverzeichnis – Index Seminum – des Botanischen Gartens der Karl-Franzens-Universität Graz, Samenernte 2016. – 54 S., Karl-Franzens-Universität Graz. Personalstand des Botanischen Gartens Graz: Institutsleiter: Ao. Univ.-Prof. Mag. Dr. Helmut MAYRHOFER Wissenschaftlicher Gartenleiter: Dr. Christian BERG Gartenverwalter: Jonathan WILFLING, B. Sc. Gärtnermeister: Friedrich STEFFAN GärtnerInnen: Doris ADAM-LACKNER Viola BONGERS Magarete HIDEN Franz HÖDL Kurt MARQUART Franz STIEBER Ulrike STRAUSSBERGER Monika GABER Gartenarbeiter: Philip FRIEDL René MICHALSKI Oliver KROPIWNICKI Gärtnerlehrlinge: Gabriel Buchmann (1. Lehrjahr) Bahram EMAMI (3. Lehrjahr) Mario MARX (3. Lehrjahr) 3 Inhaltsverzeichnis / Contents / Table des matières Abkürzungen / List of abbreviations / Abréviations -

Central European Vegetation
Plant Formations in the Central European BioProvince Peter Martin Rhind Central European Beech Woodlands Beech (Fagus sylvatica) woods form the natural climax over much of Central Europe where the soils are relatively dry and can extend well into the uplands in the more southern zones. In the north, however, around Sweden it is confined to the lowlands. Beech woodlands are often open with a poorly developed shrub layer, Characteristic ground layer species may include various helleborines such as Cephalanthera damasonium, C. longifolia and C. rubra and sedges such as Carex alba, whilst in others, grasses like Sesleria caerlea or Melica uniflora may predominate, but in some of the more acidic examples, Luzula luzuloides is likely to dominate. There are also a number of endemic ground layer species. For example, in Carpathian beech woods endemics such as Dentaria glandulosa (Brassicaceae), Symphytum cordata (Boraginaceae) and the fern Polystichum braunii (Dryopteridaceae) may be encountered. Fine examples of primeaval beech woods can be found in the limestone Alps of lower Austria including the famous ‘Rothwald’ on the southeastern slopes of Dürrentein near Lunz. These range in altitude from about 940-1480 m. Here the canopy is dominated by Fagus sylvatica together with Acer pseudoplatanus, Picea abies, Ulmus glabra, and on the more acidic soils by Abies alba. Typical shrubs include Daphne mezereum, Lonicera alpigena and Rubus hirtus. At ground level the herb layer is very rich supporting possibly up to a 100 species of vascular plants. Examples include Adenostyles alliariae, Asplenium viridis, Campanula scheuchzeri, Cardamine trifolia, Cicerbita alpina, Denteria enneaphyllos, Euphorbia amygdaloides, Galium austriacum, Homogyne alpina, Lycopodium annotinum, Mycelis muralis, Paris quadrifolia, Phyteuma spicata, Prenanthes purpurea, Senecio fuchsii, Valeriana tripteris, Veratrum album and the central European endemic Helliborus niger (Ranunculaceae). -

23Rd International Workshop of the European Vegetation Survey
23rd International Workshop of the European Vegetation Survey Ljubljana 8–12 May 2014 Book of Abstracts 23rd International Workshop of the European Vegetation Survey – Book of Abstracts Editors: Andraž Čarni, Nina Juvan, Daniela Ribeiro Issued by: Jovan Hadži Institute of Biology ZRC SAZU Anton Melik Geographical Institute ZRC SAZU Represented by: Matjaž Kuntner, Drago Perko Technical editor: Iztok Sajko Published by: ZRC Publishing House Represented by: Oto Luthar Chief Editor: Aleš Pogačnik Printrun: 260 Ljubljana, May 2014 CIP - Kataložni zapis o publikaciji Narodna in univerzitetna knjižnica, Ljubljana 581.9(4)(082) EUROPEAN Vegetation Survey. International Workshop (23 ; 2014 ; Ljubljana) Book of abstracts / 23rd International Workshop of the European Vegetation Survey, Ljubljana 8-12 May 2014 ; [editors Andraž Čarni, Nina Juvan, Daniela Ribeiro]. - Ljubljana : ZRC Publishing House, 2014 ISBN 978-961-254-693-9 1. Čarni, Andraž 273600256 Contents Welcome ..............................................................................................................5 Organising Committee ..........................................................................................6 Scientific Committee ............................................................................................6 Program ................................................................................................................7 Poster presentation ............................................................................................14 Oral presentations -

Literaturverzeichnis
Literaturverzeichnis Abaimov, A.P., 2010: Geographical Distribution and Ackerly, D.D., 2009: Evolution, origin and age of Genetics of Siberian Larch Species. In Osawa, A., line ages in the Californian and Mediterranean flo- Zyryanova, O.A., Matsuura, Y., Kajimoto, T. & ras. Journal of Biogeography 36, 1221–1233. Wein, R.W. (eds.), Permafrost Ecosystems. Sibe- Acocks, J.P.H., 1988: Veld Types of South Africa. 3rd rian Larch Forests. Ecological Studies 209, 41–58. Edition. Botanical Research Institute, Pretoria, Abbadie, L., Gignoux, J., Le Roux, X. & Lepage, M. 146 pp. (eds.), 2006: Lamto. Structure, Functioning, and Adam, P., 1990: Saltmarsh Ecology. Cambridge Uni- Dynamics of a Savanna Ecosystem. Ecological Stu- versity Press. Cambridge, 461 pp. dies 179, 415 pp. Adam, P., 1994: Australian Rainforests. Oxford Bio- Abbott, R.J. & Brochmann, C., 2003: History and geography Series No. 6 (Oxford University Press), evolution of the arctic flora: in the footsteps of Eric 308 pp. Hultén. Molecular Ecology 12, 299–313. Adam, P., 1994: Saltmarsh and mangrove. In Groves, Abbott, R.J. & Comes, H.P., 2004: Evolution in the R.H. (ed.), Australian Vegetation. 2nd Edition. Arctic: a phylogeographic analysis of the circu- Cambridge University Press, Melbourne, pp. marctic plant Saxifraga oppositifolia (Purple Saxi- 395–435. frage). New Phytologist 161, 211–224. Adame, M.F., Neil, D., Wright, S.F. & Lovelock, C.E., Abbott, R.J., Chapman, H.M., Crawford, R.M.M. & 2010: Sedimentation within and among mangrove Forbes, D.G., 1995: Molecular diversity and deri- forests along a gradient of geomorphological set- vations of populations of Silene acaulis and Saxi- tings. -

A Guide to Frequent and Typical Plant Communities of the European Alps
- Alpine Ecology and Environments A guide to frequent and typical plant communities of the European Alps Guide to the virtual excursion in lesson B1 (Alpine plant biodiversity) Peter M. Kammer and Adrian Möhl (illustrations) – Alpine Ecology and Environments B1 – Alpine plant biodiversity Preface This guide provides an overview over the most frequent, widely distributed, and characteristic plant communities of the European Alps; each of them occurring under different growth conditions. It serves as the basic document for the virtual excursion offered in lesson B1 (Alpine plant biodiversity) of the ALPECOLe course. Naturally, the guide can also be helpful for a real excursion in the field! By following the road map, that begins on page 3, you can determine the plant community you are looking at. Communities you have to know for the final test are indicated with bold frames in the road maps. On the portrait sheets you will find a short description of each plant community. Here, the names of communities you should know are underlined. The portrait sheets are structured as follows: • After the English name of the community the corresponding phytosociological units are in- dicated, i.e. the association (Ass.) and/or the alliance (All.). The names of the units follow El- lenberg (1996) and Grabherr & Mucina (1993). • The paragraph “site characteristics” provides information on the altitudinal occurrence of the community, its topographical situation, the types of substrata, specific climate conditions, the duration of snow-cover, as well as on the nature of the soil. Where appropriate, specifications on the agricultural management form are given. • In the section “stand characteristics” the horizontal and vertical structure of the community is described. -

Ex Situ Conservation of Croatian Threatened and Statutorily Protected Plant Species in the Botanical Garden of the Faculty of Science, University of Zagreb (Croatia)
NAT. CROAT. VOL. 22 No 2 343–362 ZAGREB December 31, 2013 professional paper / stručni rad MEETING TARGET EIGHT – EX SITU CONSERVATION OF CROATIAN THREATENED AND STATUTORILY PROTECTED PLANT SPECIES IN THE BOTANICAL GARDEN OF THE FACULTY OF SCIENCE, UNIVERSITY OF ZAGREB (CROATIA) Dubravka Sandev*, Darko Mihelj & Sanja Kovačić Botanical Garden, Division of Biology, Faculty of Science, University of Zagreb, Marulićev trg 9a, HR-10000 Zagreb, Croatia (*e-mail: [email protected]) Sandev, D., Mihelj, D. & Kovačić, S.: Meeting Target Eight – Ex situ conservation of Croatian threatened and statutorily protected plant species in the Botanical Garden of the Faculty of Scien- ce, University of Zagreb (Croatia). Nat. Croat., Vol. 22, No. 2, 343–362, 2013, Zagreb. According to „Target 8” of Global Strategy for Plant Conservation (GSPC, 2011-2020), at least 75% of threatened plant species must be preserved in ex situ garden-collections, preferably in the countries of their origin, until 2020. The aim of the present study was to examine the list of Croatian threatened and statutorily protected plant species in ex situ conservation of the Botanical Garden (Faculty of Sci- ence, University of Zagreb). Out of 668 indigenous taxa of the Garden’s native-plant collections, we found 208 strictly protected and protected species, which is 12.7% of all statutorily protected taxa in Croatia. In the categories RE, CR, EN and VU there are 31 species, constituting 13.1% of threatened taxa in Croatia in those categories. In all categories, we found 80 taxa listed in the Red Book of Vascular flora of Croatia, 24 taxa are identified in the European Red List of Vascular Plants, while 12 taxa are found in the IUCN-Red List of Threatened Species in the World. -

Predicting Spatial Patterns of Plant Biodiversity: from Species to Communities
Département d’écologie et d’évolution Predicting spatial patterns of plant biodiversity: from species to communities Thèse de doctorat ès sciences de la vie (PhD) Présentée à la Faculté de biologie et médecine de l’Université de Lausanne Par Anne Dubuis Master en biologie de l’Université de Lausanne Jury Prof. Stefan Kunz, Président Prof. Antoine Guisan, Directeur de thèse Dr. Pascal Vittoz, Expert Prof. Jean-Claude Gégout, Expert Prof. Miska Luoto, Expert Lausanne 2013 SUMMARY Understanding the distribution and composition of species assemblages and being able to predict them in space and time are highly important tasks to investigate the fate of biodiversity in the current global changes context. Species distribution models are tools that have proven useful to predict the potential distribution of species by relating their occurrences to environmental variables. Species assemblages can then be predicted by combining the prediction of individual species models. In the first part of my thesis, I tested the importance of new environmental predictors to improve species distribution prediction. I showed that edaphic variables, above all soil pH and nitrogen content could be important in species distribution models. In a second chapter, I tested the influence of different resolution of predictors on the predictive ability of species distribution models. I showed that fine resolution predictors could ameliorate the models for some species by giving a better estimation of the micro-topographic condition that species tolerate, but that fine resolution predictors for climatic factors still need to be ameliorated. The second goal of my thesis was to test the ability of empirical models to predict species assemblages’ characteristics such as species richness or functional attributes. -

Dissertation / Doctoral Thesis
DISSERTATION / DOCTORAL THESIS Titel der Dissertation /Title of the Doctoral Thesis „ Forecasting Plant Invasions in Europe: Effects of Species Traits, Horticultural Use and Climate Change. “ verfasst von / submitted by Günther Klonner, BSc MSc angestrebter akademischer Grad / in partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) Wien, 2017 / Vienna 2017 Studienkennzahl lt. Studienblatt / A 794 685 437 degree programme code as it appears on the student record sheet: Dissertationsgebiet lt. Studienblatt / Biologie field of study as it appears on the student record sheet: Betreut von / Supervisor: Univ.-Prof. Mag. Dr. Stefan Dullinger “Whatever makes the past, the distant, or the future, predominate over the present, advances us in the dignity of thinking beings.” Samuel Johnson (1791) “In the 1950s, the planet still had isolated islands, in both geographical and cultural terms - lands of unique mysteries, societies, and resources. By the end of the 20th century, expanding numbers of people, powerful technology, and economic demands had linked Earth’s formerly isolated, relatively non-industrialized places with highly developed ones into an expansive and complex network of ideas, materials, and wealth.” Lutz Warren and Kieffer (2010) ACKNOWLEDGEMENT As for many others, the last few years have been an up and down, however, I made it through thanks to my family, friends and colleagues without whom this wouldn’t have been possible. To the love of my life, my wife Christine Schönberger: because you are my support, advice and courage. Because we share the same dreams. Many Thanks! My brother and one of my best friends, Dietmar, who provided me through emotional support in many situations and shares my love to mountains and sports: Thank you! I am also grateful to my other family members, especially my parents Ingeborg and Martin, who were always keen to know what I was doing and how I was proceeding. -

Medicinal, Biological and Phytochemical Properties of Gentiana Species
31/08/2021 Medicinal, biological and phytochemical properties of Gentiana species J Tradit Complement Med. 2017 Oct; 7(4): 400–408. PMCID: PMC5634738 Published online 2017 Jan 28. PMID: 29034186 doi: 10.1016/j.jtcme.2016.12.013: 10.1016/j.jtcme.2016.12.013 Medicinal, biological and phytochemical properties of Gentiana species Fatemeh Mirzaee,a Amirsaeed Hosseini,b Hossein Bakhshi Jouybari,a Ali Davoodi,a and Mohammad Azadbakhta,∗ aDepartment of Pharmacognosy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran bTraditional and Complementary Medicine Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran Mohammad Azadbakht: [email protected] ∗Corresponding author. [email protected] Received 2016 Oct 19; Revised 2016 Dec 18; Accepted 2016 Dec 30. Copyright © 2017 Center for Food and Biomolecules, National Taiwan University. Production and hosting by Elsevier Taiwan LLC. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Abstract Gentiana, a cosmopolitan and important genus of the Gentianaceae family, comprises 400 species distributed among the world. Based on the studies of Iranian traditional medicine texts, there are some promising bioactivities for this genus that is unknown in modern medicine and some of them are still the basis of new remedies. In traditional medicine texts, Gentiana's different exclusive forms of preparations are effective for treatment of some disorders such as menstrual over-bleeding, conjunctivitis, vitiligo, animals venom poisoning, injuries, infected wounds, pain and swelling of liver, spleen, stomach and sprains of muscles. There are some activities that are the same in traditional and modern medicine such as anti-inflammatory, hepatoprotective and diuretic effects. -

2021 Plant List
Wrightman Alpines Nursery 2021Plant List A343 Acantholimon alatavicum Sun prodMessage: $12 Perfect cushions of glaucous green needle-like leaves, host spikes of pale pink flowers 5 to 10cm above. M. Pavelka collection from rocky north-eastern slopes at 2300m; Tian-Shan, Kyrgyzstan. A196 Acantholimon trojanum Sun prodMessage: $12 Dense, silvery cushions with big rose/pink flowers ~10cm tall; from Kaz Dag, Turkey: 1600m. A322 Achillea oxyloba ssp. schurii Sun prodMessage: $10 Simple white daisy flowers (10-15cm high) on compact cushion of green, finely cut leaves. From the stony slopes of Bucegi Mts, Romania, 2300m; Pavelka collection. A072 Achillea x lewisii 'King Edward' Sun prodMessage: $9 Toothed, wooly, gray-green leaves form mats with 15cm flower stems that bear corms of pale yellow flowers, which age to cream. A344 Aconitum cf. brachypodum Sun prodMessage: $11 Spikes covered in purple-blue blooms, grows to about 40cm tall from a tuft of pinnatisect leaves. Jurasek collection from SW Qinghai at 4300m. A105 Adenophora takedae v. howozana Part-sun prodMessage: $10 Campanula relative from Japan; slender arching branches with narrow, toothed leaves. Blue violet bells in very late in August-September; 15cm. A291 Aethionema armenum Sun prodMessage: $9 Compact 10cm evergreen mound of soft pink blooms. Easy to grow and will gently self seed about. Jurasek collection from Turkey. A280 Aethionema saxatilis Sun prodMessage: $9 Blue-green, thick (almost succulent like) leaves form a low mat of 10-15cm. Flowers are usually pink, but there is variability with white and mauve as well. Jurasek collection, 1700m Greece. A198 Aethionema subulatum Sun Part-sunprodMessage: $10 Compact tufts of woody stems with bluish leaves; large rose/pink flowers on short scapes.