Seasonal Dynamics of the Marine CO System in Adventfjorden, a West
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Climate in Svalbard 2100
M-1242 | 2018 Climate in Svalbard 2100 – a knowledge base for climate adaptation NCCS report no. 1/2019 Photo: Ketil Isaksen, MET Norway Editors I.Hanssen-Bauer, E.J.Førland, H.Hisdal, S.Mayer, A.B.Sandø, A.Sorteberg CLIMATE IN SVALBARD 2100 CLIMATE IN SVALBARD 2100 Commissioned by Title: Date Climate in Svalbard 2100 January 2019 – a knowledge base for climate adaptation ISSN nr. Rapport nr. 2387-3027 1/2019 Authors Classification Editors: I.Hanssen-Bauer1,12, E.J.Førland1,12, H.Hisdal2,12, Free S.Mayer3,12,13, A.B.Sandø5,13, A.Sorteberg4,13 Clients Authors: M.Adakudlu3,13, J.Andresen2, J.Bakke4,13, S.Beldring2,12, R.Benestad1, W. Bilt4,13, J.Bogen2, C.Borstad6, Norwegian Environment Agency (Miljødirektoratet) K.Breili9, Ø.Breivik1,4, K.Y.Børsheim5,13, H.H.Christiansen6, A.Dobler1, R.Engeset2, R.Frauenfelder7, S.Gerland10, H.M.Gjelten1, J.Gundersen2, K.Isaksen1,12, C.Jaedicke7, H.Kierulf9, J.Kohler10, H.Li2,12, J.Lutz1,12, K.Melvold2,12, Client’s reference 1,12 4,6 2,12 5,8,13 A.Mezghani , F.Nilsen , I.B.Nilsen , J.E.Ø.Nilsen , http://www.miljodirektoratet.no/M1242 O. Pavlova10, O.Ravndal9, B.Risebrobakken3,13, T.Saloranta2, S.Sandven6,8,13, T.V.Schuler6,11, M.J.R.Simpson9, M.Skogen5,13, L.H.Smedsrud4,6,13, M.Sund2, D. Vikhamar-Schuler1,2,12, S.Westermann11, W.K.Wong2,12 Affiliations: See Acknowledgements! Abstract The Norwegian Centre for Climate Services (NCCS) is collaboration between the Norwegian Meteorological In- This report was commissioned by the Norwegian Environment Agency in order to provide basic information for use stitute, the Norwegian Water Resources and Energy Directorate, Norwegian Research Centre and the Bjerknes in climate change adaptation in Svalbard. -

Terrestrial Inputs Govern Spatial Distribution of Polychlorinated Biphenyls (Pcbs) and Hexachlorobenzene (HCB) in an Arctic Fjord System (Isfjorden, Svalbard)*
Environmental Pollution 281 (2021) 116963 Contents lists available at ScienceDirect Environmental Pollution journal homepage: www.elsevier.com/locate/envpol Terrestrial inputs govern spatial distribution of polychlorinated biphenyls (PCBs) and hexachlorobenzene (HCB) in an Arctic fjord system (Isfjorden, Svalbard)* * Sverre Johansen a, b, c, Amanda Poste a, Ian Allan c, Anita Evenset d, e, Pernilla Carlsson a, a Norwegian Institute for Water Research, Tromsø, Norway b Norwegian University of Life Sciences, Ås, Norway c Norwegian Institute for Water Research, Oslo, Norway d Akvaplan-niva, Tromsø, Norway e UiT, The Arctic University of Norway, Tromsø, Norway article info abstract Article history: Considerable amounts of previously deposited persistent organic pollutants (POPs) are stored in the Received 20 July 2020 Arctic cryosphere. Transport of freshwater and terrestrial material to the Arctic Ocean is increasing due to Received in revised form ongoing climate change and the impact this has on POPs in marine receiving systems is unknown This 11 March 2021 study has investigated how secondary sources of POPs from land influence the occurrence and fate of Accepted 13 March 2021 POPs in an Arctic coastal marine system. Available online 17 March 2021 Passive sampling of water and sampling of riverine suspended particulate matter (SPM) and marine sediments for analysis of polychlorinated biphenyls (PCBs) and hexachlorobenzene (HCB) was carried out Keywords: Particle transport in rivers and their receiving fjords in Isfjorden system in Svalbard. Riverine SPM had low contaminant < S e Terrestrial runoff concentrations ( level of detection-28 pg/g dw PCB14,16 100 pg/g dw HCB) compared to outer marine Environmental contaminants sediments 630-880 pg/g dw SPCB14,530e770 pg/g dw HCB). -
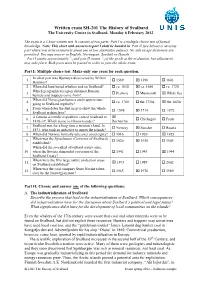
Written Exam SH-201 the History of Svalbard the University Centre in Svalbard, Monday 6 February 2012
Written exam SH-201 The History of Svalbard The University Centre in Svalbard, Monday 6 February 2012 The exam is a 3 hour written test. It consists of two parts: Part I is a multiple choice test of factual knowledge. Note: This sheet with answers to part I shall be handed in. Part II (see below) is an essay part where you write extensively about one of two alternative subjects. No aids except dictionary are permitted. You may answer in English, Norwegian, Swedish or Danish. 1 2 Part I counts approximately /3 and part II counts /3 of the grade at the evaluation, but adjustment may take place. Both parts must be passed in order to pass the whole exam. Part I: Multiple choice test. Make only one cross for each question. In what year was Bjørnøya discovered by Willem 1. 1569 1596 1603 Barentsz? 2. When did land-based whaling end on Svalbard? ca. 1630 ca. 1680 ca. 1720 Which geographical region did most Russian 3. Pechora Murmansk White Sea hunters and trappers come from? When did Norwegian hunters and trappers start 4. ca. 1700 the 1750s the 1820s going to Svalbard regularly? From when dates the first map to show the whole 5. 1598 1714 1872 Svalbard archipelago? A famous scientific expedition visited Svalbard in 6. Chichagov Fram 1838–39. Which name is it known under? Recherche Svalbard was for a long time a no man’s land. In 7. Norway Sweden Russia 1871, who took an initiative to annex the islands? 8. When did Norway formally take over sovereignty? 1916 1920 1925 When was the Sysselmann (Governor of Svalbard) 9. -

Svalbard 2015–2016 Meld
Norwegian Ministry of Justice and Public Security Published by: Norwegian Ministry of Justice and Public Security Public institutions may order additional copies from: Norwegian Government Security and Service Organisation E-mail: [email protected] Internet: www.publikasjoner.dep.no KET T Meld. St. 32 (2015–2016) Report to the Storting (white paper) Telephone: + 47 222 40 000 ER RY M K Ø K J E L R I I Photo: Longyearbyen, Tommy Dahl Markussen M 0 Print: 07 PrintMedia AS 7 9 7 P 3 R 0 I 1 08/2017 – Impression 1000 N 4 TM 0 EDIA – 2 Svalbard 2015–2016 Meld. St. 32 (2015–2016) Report to the Storting (white paper) 1 Svalbard Meld. St. 32 (2015–2016) Report to the Storting (white paper) Svalbard Translation from Norwegian. For information only. Table of Contents 1 Summary ........................................ 5 6Longyearbyen .............................. 39 1.1 A predictable Svalbard policy ........ 5 6.1 Introduction .................................... 39 1.2 Contents of each chapter ............... 6 6.2 Areas for further development ..... 40 1.3 Full overview of measures ............. 8 6.2.1 Tourism: Longyearbyen and surrounding areas .......................... 41 2Background .................................. 11 6.2.2 Relocation of public-sector jobs .... 43 2.1 Introduction .................................... 11 6.2.3 Port development ........................... 44 2.2 Main policy objectives for Svalbard 11 6.2.4 Svalbard Science Centre ............... 45 2.3 Svalbard in general ........................ 12 6.2.5 Land development in Longyearbyen ................................ 46 3 Framework under international 6.2.6 Energy supply ................................ 46 law .................................................... 17 6.2.7 Water supply .................................. 47 3.1 Norwegian sovereignty .................. 17 6.3 Provision of services ..................... -

Gatekart Over Longyearbyen
6YkZciYVaZc K:,K:>:CÇ 6YkZci[_dgYZc K:>)%%Æ<GJ H_³h`gZciZc K:>'(- K:>'(+ 7n`V^V$L]Vg[ H_³dbgYZi K:>*%% K:>'() <gjkZYVaZc K:>'%%Æ=>AB6GG:@HI:CHK:>Ç K:>(%+ 7_³gcYVaZc K:>'(' K :> (%% K:>(%. B6K:>:CÇ<6C<K:>I>A;ANEA6HH:C K:>(%*Æ7JG $;DDIE6I=ID6>GEDGI K:>(%, K K:>'(% :> *% (B :A@ H`_²g^c\V :K: >:C Adc\nZVgZakV K:>(%( A^V K:>(%% K:>''- K:>*%% + K:>'' <VbaZ K:>(%' Adc\nZVgWnZc K:>'') K:>*%& K :> ''' K:>''% K:>&%% K:>(%% K:>'&- K:>'&+ K:>'&* K:>'&) K:>'&( K =Vj\Zc : > K:>&%' ' & % K:>&%%Æ9G6BB:CHK:>:CÇ K:>&%+ <gjkZ'W ?jaZc^hhZ\gjkV CnWnZc HkZgYgjeWnZc K:>&%% <gV[^h`WZVgWZ^YZahZ/HkVaWVgYGZ^hZa^k6H^hVbVgWZ^YbZYAjcYWaVYBZY^V6H!Igdbh³#@Vgi\gjccaV\Adc\nZVgWnZcad`VahingZ OVERNATTING ACCOMMODATION RaBi’s Bua HELSETJENESTER HEALTH SERVICES Arctic Adventures Rebekkas Gave & Suvenir Apotek Chemist/pharmacy Basecamp Spitsbergen Skinnboden Arctic Products Fysioterapeut Physiotherapis Gjestehuset 102 Sport 1 Svalbard Longyearbyen Sykehus Hospital Longyearbyen Camping Sportscenteret PolarLegeSenteret Medical Centre Mary-Ann’s Polarrigg Svalbard Arctic Sport/Skandinavisk Høyfjellsutstyr Tannlege Dentist Radisson SAS Polar Hotel Spitsbergen Svalbardbutikken (Coop Svalbard) Spitsbergen Guesthouse Svalbardbutikken Lyd & Bilde SEVERDIGHETER ATTRACTIONS Spitsbergen Hotel Atelier Aino Artist’s Workshop Svalbard Villmarkssenter TJENESTER SERVICES Barentszhuset The Barentsz House Arctic Autorent Car rental Galleri Svalbard Svalbard Art Gallery MAT OG DRIKKE FOOD & DRINK Bydrift Longyearbyen Gruve 3 Mine no. 3 Barentsz Pub & Spiseri Kultur- og fritidsforetak KF Kirkegård -

Review Article: Permafrost Trapped Natural Gas in Svalbard, Norway
https://doi.org/10.5194/tc-2021-226 Preprint. Discussion started: 9 August 2021 c Author(s) 2021. CC BY 4.0 License. 1 Review Article: Permafrost Trapped Natural Gas in 2 Svalbard, Norway 3 Authors: Thomas Birchall*1, 2, Malte Jochmann1, 3, Peter Betlem1, 2, Kim Senger1, Andrew 4 Hodson1, Snorre Olaussen1 5 1Department of Arctic Geology, The University Centre in Svalbard, P.O. Box 156, N-9171 Longyearbyen, 6 Svalbard, Norway 7 2Department of Geosciences, University of Oslo, P.O. Box 1047, Blindern, 0316 Oslo, Norway 8 3Store Norske Spitsbergen Kulkompani AS, Vei 610 2, 9170 Longyearbyen, Svalbard. Norway 9 *Correspondence to: Thomas Birchall ([email protected]) 10 11 Abstract. Permafrost has become an increasingly important subject in the High Arctic archipelago of Svalbard. 12 However, whilst the uppermost permafrost intervals have been well studied, the processes at its base and the 13 impacts of the underlying geology have been largely overlooked. More than a century of coal, hydrocarbon and 14 scientific drilling through the permafrost interval shows that accumulations of natural gas trapped at the base 15 permafrost is common. They exist throughout Svalbard in several stratigraphic intervals and show both 16 thermogenic and biogenic origins. These accumulations combined with the relatively young permafrost age 17 indicate gas migration, driven by isostatic rebound, is presently ongoing throughout Svalbard. The accumulation 18 sizes are uncertain, but one case demonstrably produced several million cubic metres of gas over eight years. Gas 19 encountered in two boreholes on the island of Hopen appears to be situated in the gas hydrate stability zone and 20 thusly extremely voluminous. -

Foundation of Infrastructure in Pyramiden and Longyearbyen
University Centre in Svalbard, AT‐301 1 Foundation of infrastructures in Pyramiden and Longyearbyen Foundation of infrastructure in Pyramiden and Longyearbyen A comparative study of the types of foundation in the two cities, building damages, and a discussion of reasons for failures: 26. August 2009 Christian Katlein and Kristoffer Hallberg 1. Introduction This report tries to give an overview about the foundations of infrastructures in two cities in Svalbard. Longyearbyen is the main Norwegian city situated in the southern part of the Isfjorden at the end of the Adventfjorden. It runs infrastructures like a normal city containing buildings for living, shopping centres, administrative buildings, airport, roads, pipelines and a lot more. The foundations of that infrastructure will be compared to that one left over in Pyramiden, which is an abandoned Russian mining city in the northern part of Isfjorden in the Billefjorden. Pyramiden was abandoned in 1998 and suffers from a continuous decay. In the last ten years no maintenance work was done until the Russian administration planned to reuse the city again recently. We will have a look on the different types of foundations and some occurring damages also discussing reasons for failure. 2.Key Concepts Frozen ground engineering includes construction and excavation works on permanent frozen soil, seasonal thawed soil or a combination of those two. Frozen permafrost soil at low temperature often shows greater strength than permafrost at higher temperatures. Soil with less strength is associated with poor drainage and thawing. Many engineering tasks such as foundation take advantage of the mechanical properties of frozen soil but also suffer from the effects associated with thawing and creep. -

The Svalbard Airport Temperature Series
Bulletin of Geography – physical geography series No 3/2010: 5–25 https://www.doi.org/10.2478/bgeo-2010-0001 Øyvind Nordli Norwegian Meteorological Institute, PO Box 43, Blindern, NO-0313 Oslo, Norway [email protected] THE SVALBARD AIRPORT TEMPERATURE SERIES Abstract: In the Isfjorden region of Spitsbergen in the Svalbard archipel- ago, the air temperature has been observed continuously at different sites since 1911 (except for a break during WW II). The thermal conditions at these various sites turned out to be different so that nesting the many se- ries together in one composite time series would produce an inhomogenous long-term series. By using the SNHT (Standard Normal Homogeneity Test) the differences between the sites were assessed and the series adjusted ac- cordingly. This resulted in an homogenised, composite series mainly from Green Harbour (Finneset in Grønfjorden), Barentsburg (also in Grønfjor- den), Longyearbyen and the current observation site at Svalbard Airport. A striking feature in the series is a pronounced, abrupt change from cold temperature in the 1910s to warmth in the 1930s, when temperature reached a local maximum. This event is called the early 20th century warming. There- after the temperature decreased to a local minimum in the 1960s before the start of another increase that still seems to be ongoing. For the whole series, statistically significant positive trends were detected by the Mann-Kendall test for annual and seasonal values (except for winter). Quite often the Norwegian Meteorological Institute receives queries about long-term temperature series from Svalbard. Hopefully, the Svalbard Airport composite series will fulfil this demand for data. -

Spitsbergen Nordaustlandet Polhavet Barentshavet
5°0'0"E 10°0'0"E 15°0'0"E 20°0'0"E 25°0'0"E 30°0'0"E 35°0'0"E 81°0'0"N Polhavet Prins Oscars Land Orvin Land Vesle Tavleøya Gustav V Land Nordaustlandet Karl XII-øya Phippsøya Sjuøyane Gustav Adolf Land 80°0'0"N Martensøya Parryøya Kvitøyjøkulen Waldenøya Foynøya Nordkappsundet Kvitøya Repøyane Castrénøyane 434 433 ZorgdragerfjordenDuvefjorden Snøtoppen Nordenskiöldbukta Scoresbyøya Wrighttoppen Brennevinsfjorden 432 Laponiahalvøya Damflya Lågøya Storøya Kvitøyrenna Storøysundet Botniahalvøya Sabinebukta Orvin Land 437 Rijpfjorden Prins Lady Franklinfjorden Maudbreen Oscars Franklinsundet Worsleybreen Sverdrupisen 80°0'0"N Land Andrée Land Rijpbreen Albert I Land Norskebanken Ny-Friesland Storsteinhalvøya Olav V Land Franklinbreane James I Land Oscar II Land 401 Hinlopenrenna Haakon VII Land Gustav V Moffen Celsiusberget Murchisonfjorden Land Rijpdalen Søre Russøya Vestfonna Harald V Land Mosselhalvøya Austfonna Sorgfjorden 436 Heclahuken Gotiahalvøya Nordaustlandet Harald V Land Fuglesongen Oxfordhalvøya Breibogen Bragebreen Etonbreen IdunfjelletWahlenbergfjorden 435 Amsterdamøya Reinsdyrflya 427 Raudfjorden Balberget Hartogbukta Danskøya Vasahalvøya Smeerenburgfjorden Valhallfonna 79°0'0"N Ben Nevis 428 Woodfjorden Reuschhalvøya Palanderbukta Gustav Adolf Liefdefjorden Magdalenefjorden Åsgardfonna Albert I Glitnefonna Roosfjella Wijdefjorden Land 430 431 Hoelhalvøya Scaniahalvøya Land Bockfjorden Lomfjorden Hinlopenstretet Vibehøgdene Lomfjordhalvøya Svartstupa Monacobreen 429 Seidfjellet Svartknausflya Bråsvellbreen 402 Lomfjella Vibebukta -

Twenty of the Most Thermophilous Vascular Plant Species in Svalbard and Their Conservation State
Twenty of the most thermophilous vascular plant species in Svalbard and their conservation state Torstein Engelskjøn, Leidulf Lund & Inger Greve Alsos An aim for conservation in Norway is preserving the Svalbard archi- pelago as one of the least disturbed areas in the Arctic. Information on local distribution, population sizes and ecology is summarized for 20 thermophilous vascular plant species. The need for conservation of north- ern, marginal populations in Svalbard is reviewed, using World Conser- vation Union categories and criteria at a regional scale. Thirteen species reach their northernmost distribution in Svalbard, the remaining seven in the western Arctic. Nine species have 1 - 8 populations in Svalbard and are assigned to Red List categories endangered or critically endangered: Campanula rotundifolia, Euphrasia frigida, Juncus castaneus, Kobresia simpliciuscula, Rubus chamaemorus, Alchemilla glomerulans, Ranuncu- lus wilanderi, Salix lanata and Vaccinium uliginosum, the last four spe- cies needing immediate protective measures. Five species are classifi ed as vulnerable: Betula nana, Carex marina ssp. pseudolagopina, Luzula wahlenbergii, Ranunculus arcticus and Ranunculus pallasii. Six species are considered at lower risk: Calamagrostis stricta, Empetrum nigrum ssp. hermaphroditum, Hippuris vulgaris (only occurring on Bjørnøya), Juncus triglumis, Ranunculus lapponicus and Rhodiola rosea. The warmer Inner Arctic Fjord Zone of Spitsbergen supports most of the 20 target species and is of particular importance for conservation. Endan- gered or vulnerable species were found in a variety of edaphic conditions; thus, several kinds of habitats need protection. T. Engelskjøn, I. G. Alsos, Tromsø Museum, University of Tromsø, NO-9037 Tromsø, Norway, torstein@ tmu.uit.no; L. Lund, Phytotron, University of Tromsø, NO-9037 Tromsø, Norway. -

2000 Svalbard Workshop Report (PDF 2.5
Opportunities for Collaboration Between the United States and Norway in Arctic Research A Workshop Report Arctic Research Consortium of the U.S. (ARCUS) 600 University Avenue, Suite 1 Fairbanks, Alaska, 99709 USA Phone: 907/474-1600 Fax: 907/474-1604 http://www.arcus.org This publication may be cited as: Opportunities for Collaboration Between the United States and Norway in Arctic Research: A Workshop Report. The Arctic Research Consortium of the U.S. (ARCUS), Fairbanks, Alaska, USA. August 2000. 102 pp. This report is published by ARCUS with funding provided by the National Science Founda- tion (NSF) under Cooperative Agreement OPP-9727899. Any opinions, findings, and conclu- sions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF. Table of Contents Executive Summary v Summary of Recommendations viii Chapter 1. Research in Svalbard in a Global Context 1 ii Justification and Process 1 Current Arctic Research in a Global Context 2 Svalbard as a Research Platform 2 Longyearbyen 4 Ny-Ålesund 4 Arctic Research Policy 5 Norwegian Arctic Research Policy 6 U.S. Arctic Research Policy 7 Science Priorities for U.S.-Norwegian Collaboration in Svalbard 7 Multidisciplinary Themes 7 Specific Disciplinary Topics 14 Chapter 2. Research Support Infrastructure 25 Circumpolar Research Infrastructure 25 Svalbard’s Value and Potential 25 Longyearbyen 26 Ny-Ålesund 28 Specific Research Facilities on Svalbard 32 Chapter 3. Recommendations for Investments to Improve Collaborative Opportunities -

UNIS Annual Report 2020
ANNUAL REPORT 2020 2 | ANNUAL REPORT 2020 FROM THE DIRECTOR 4 EXCERPT FROM THE BOARD OF DIRECTORS' REPORT 2020 5 STATISTICS 9 PROFIT AND LOSS ACCOUNT 2020 10 BALANCE SHEET 31.12.2020 11 ARCTIC BIOLOGY 12 BIOCEED 19 ARCTIC GEOLOGY 20 ARCTIC GEOPHYSICS 26 ARCTIC TECHNOLOGY 32 ARCTIC SAFETY CENTRE 38 SCIENTIFIC PUBLICATIONS 2020 40 Front page | May 2020: Performing drone fieldwork at Fridtjovbreen. Photo: Christina Hess. Editor | Eva Therese Jenssen. ANNUAL REPORT 2020 | 3 NY-ÅLESUND LONGYEARBYEN BARENTSBURG SVEA HORNSUND SVALBARD February 2020: Longyearbyen seen from Gruvefjellet. Photo: Sil Schuuring/UNIS. 4 | ANNUAL REPORT 2020 FROM THE DIRECTOR With the outbreak of the Covid-19 pandemic, 2020 became challenging year for both staff and students. On 12 March all universities in Norway were closed down. UNIS had at that time 180 regular course students. Our main task became to ensure that all of our students would receive their study credits and maintain the planned study progress. The lectures and seminars went digital. Thanks to our skilled staff that quickly adapted to digital learning despite working from home. The exam success rate of 97% speaks for itself. Jøran Moen is managing director of UNIS. Photo: Eva Therese Jenssen/UNIS. meaning that the learning outcomes cannot be achieved All courses at UNIS have a mandatory field component, of environmental and societal consequences of rapid decidedwithout tofieldwork. cancel all Due courses to infection for the controlrest of themeasures year. A it warmingUNIS contribute in the Arctic, significantly and through to the bothunderstanding research and healthwas not adviser possible with to guaranteeparticular classesresponsibility in the field, for infection and UNIS control measures was hired to develop routines for all the UN´s sustainable development goals.