Canis Aureus) in Agro-Ecosystems of Bangladesh
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolutionary Biology of the Genus Rattus: Profile of an Archetypal Rodent Pest
Bromadiolone resistance does not respond to absence of anticoagulants in experimental populations of Norway rats. Heiberg, A.C.; Leirs, H.; Siegismund, Hans Redlef Published in: <em>Rats, Mice and People: Rodent Biology and Management</em> Publication date: 2003 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Heiberg, A. C., Leirs, H., & Siegismund, H. R. (2003). Bromadiolone resistance does not respond to absence of anticoagulants in experimental populations of Norway rats. In G. R. Singleton, L. A. Hinds, C. J. Krebs, & D. M. Spratt (Eds.), Rats, Mice and People: Rodent Biology and Management (Vol. 96, pp. 461-464). Download date: 27. Sep. 2021 SYMPOSIUM 7: MANAGEMENT—URBAN RODENTS AND RODENTICIDE RESISTANCE This file forms part of ACIAR Monograph 96, Rats, mice and people: rodent biology and management. The other parts of Monograph 96 can be downloaded from <www.aciar.gov.au>. © Australian Centre for International Agricultural Research 2003 Grant R. Singleton, Lyn A. Hinds, Charles J. Krebs and Dave M. Spratt, 2003. Rats, mice and people: rodent biology and management. ACIAR Monograph No. 96, 564p. ISBN 1 86320 357 5 [electronic version] ISSN 1447-090X [electronic version] Technical editing and production by Clarus Design, Canberra 431 Ecological perspectives on the management of commensal rodents David P. Cowan, Roger J. Quy* and Mark S. Lambert Central Science Laboratory, Sand Hutton, York YO41 1LZ, UNITED KINGDOM *Corresponding author, email: [email protected] Abstract. The need to control Norway rats in the United Kingdom has led to heavy reliance on rodenticides, particu- larly because alternative methods do not reduce rat numbers as quickly or as efficiently. -

The Yellow Mongoose Cynictis Penicillata in the Great Fish River Reserve (Eastern Cape, South Africa)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) Spatio-temporal ecology of the yellow mongoose Cynictis penicillata in the Great Fish River Reserve (Eastern Cape, South Africa) By Owen Akhona Mbatyoti A dissertation submitted in fulfilment of the requirements for the degree of MASTER OF SCIENCE (ZOOLOGY) in the Faculty of Science and Agriculture at the University of Fort Hare August 2012 Supervisor: Dr Emmanuel Do Linh San DECLARATION 1. This is to declare that this dissertation entitled “Spatio-temporal ecology of the yellow mongoose Cynictis penicillata in the Great Fish River Reserve (Eastern Cape, South Africa)” is my own work and has not been previously submitted to another institute. 2. I know that plagiarism means taking and using the ideas, writings, work or inventions of another person as if they were one’s own. I know that plagiarism not only includes verbatim copying, but also extensive use of another person’s ideas without proper acknowledgement (which sometimes includes the use of quotation marks). I know that plagiarism covers this sort of material found in textual sources (e.g. books, journal articles and scientific reports) and from the Internet. 3. I acknowledge and understand that plagiarism is wrong. 4. I understand that my research must be accurately referenced. I have followed the academic rules and conventions concerning referencing, citation and the use of quotations. 5. I have not allowed, nor will I in the future allow, anyone to copy my work with the intention of passing it off as their own work. -

Use of Analgesics in Exotic Felids Edward C. Ramsay, DVM Professor, Department of Small Animal Clinical Sciences, University Of
Use of Analgesics in Exotic Felids Formatted: Centered Edward C. Ramsay, DVM Professor, Department of Small Animal Clinical Sciences, University of Tennessee, and Consulting Veterinarian, Knoxville Zoological Gardens, Knoxville, Tennessee. Corresponding address: Ed Ramsay Dept. of Sm Animal Clin Sci C247, Veterinary Teaching Hospital University of Tennessee Knoxville, TN 37996-4544 Phone: 865-755-8219 FAX: 865-974-5554 Email: [email protected] 2 Treatment of pain in domestic and non-domestic cats has been a challenge for the clinician. Many cat species are stoic and show few or very subtle external signs of pain. Additionally, the adverse effects of nonsteroidal antiinflammatory drugs (NSAIDs) in domestic cats are well documented and have discouraged many practitioners from trying novel NSAID’s in exotic felids. As in other animals, each cat’s response to pain and analgesics will vary, necessitating an individualized treatment plan. As a rule, always treat painful felids to effect, and not by rote reliance on published dosages. It is frequently necessary to try different agents and combinations to find which produces the optimal analgesic effect in exotic felids. In order to minimize adverse effects, it is desirable to work toward treatment with the lowest effective dose when treating chronic pain. Non-steroidal Antiinflammatory Drugs NSAIDs are antiinflammatory drugs which act both centrally and peripherally. The primary effects are believed to be caused by their ability to inhibit cyclooxygenase (COX) enzymes in the arachidonic acid metabolism cascade. The COX-1 isoform is regarded as constitutive (continuously expressed) and is responsible for many homeostatic processes, such as maintenance of gastric mucosal integrity, platelet function, and renal autoregulation. -

Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal
SMALL CARNIVORES IN TINJURE-MILKE-JALJALE, EASTERN NEPAL The content of this booklet can be used freely with permission for any conservation and education purpose. However we would be extremely happy to get a hard copy or soft copy of the document you have used it for. For further information: Friends of Nature Kathmandu, Nepal P.O. Box: 23491 Email: [email protected], Website: www.fonnepal.org Facebook: www.facebook.com/fonnepal2005 First Published: April, 2018 Photographs: Friends of Nature (FON), Jeevan Rai, Zaharil Dzulkafly, www.pixabay/ werner22brigitte Design: Roshan Bhandari Financial support: Rufford Small Grants, UK Authors: Jeevan Rai, Kaushal Yadav, Yadav Ghimirey, Som GC, Raju Acharya, Kamal Thapa, Laxman Prasad Poudyal and Nitesh Singh ISBN: 978-9937-0-4059-4 Acknowledgements: We are grateful to Zaharil Dzulkafly for his photographs of Marbled Cat, and Andrew Hamilton and Wildscreen for helping us get them. We are grateful to www.pixabay/werner22brigitte for giving us Binturong’s photograph. We thank Bidhan Adhikary, Thomas Robertson, and Humayra Mahmud for reviewing and providing their valuable suggestions. Preferred Citation: Rai, J., Yadav, K., Ghimirey, Y., GC, S., Acharya, R., Thapa, K., Poudyal, L.P., and Singh, N. 2018. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal. Friends of Nature, Nepal and Rufford Small Grants, UK. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal Why Protect Small Carnivores! Small carnivores are an integral part of our ecosystem. Except for a few charismatic species such as Red Panda, a general lack of research and conservation has created an information gap about them. I am optimistic that this booklet will, in a small way, be the starting journey of filling these gaps in our knowledge bank of small carnivore in Nepal. -

The Nature of Teller Coyotes Physical Description Coyotes (Canis Latrans
The Nature of Teller Coyotes Physical Description Coyotes (Canis latrans) are members of the canine family. Of the 19 subspecies, the mountain coyote is the one you’ll encounter in Teller County. They have gray, white, tan, and brown fur. The coyote in the photograph was taken from Edlowe Road. They are about the size of a medium-size dog, weighing 20 to 50 pounds. Their long, bushy tails are helpful species identifiers. Coyotes run with their tails down while domestic dogs run with tails up and wolves run with tails straight out. Life History In many areas, coyotes are solitary outside of the breeding season; but their social organization is influenced by prey size. In populations where the majority of prey are small rodents, coyotes tend to be solitary. In populations where larger animals are available (elk and deer), large groups of coyotes (packs) may form. Like other canines, coyotes do not hibernate. A male and female will pair off and remain together for several years, although they may not be life mates. Mating occurs between January and March. They establish dens abandoned by other animals, or dig one themselves. Litters of 5-7are born sightless and hairless two months after mating. Their eyes open after 10 days, and they leave the den between 8-10 weeks of age. Movement of pups from one den to another is common. The reason is unknown, but disturbance and infestation by parasites may be factors. Coyotes in captivity may live as long as 18 years, but in wild populations few coyotes live more than 6 to 8 years. -

Ecology of the Small Indian Mongoose (Herpestes Auropunctatus) in North America
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Publications Plant Health Inspection Service 2018 Ecology of the Small Indian Mongoose (Herpestes auropunctatus) in North America Are R. Berentsen USDA National Wildlife Research Center, [email protected] William C. Pitt Smithsonian Institute Robert T. Sugihara USDA/APHIS/WS/National Wildlife Research Center Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Part of the Life Sciences Commons Berentsen, Are R.; Pitt, William C.; and Sugihara, Robert T., "Ecology of the Small Indian Mongoose (Herpestes auropunctatus) in North America" (2018). USDA National Wildlife Research Center - Staff Publications. 2034. https://digitalcommons.unl.edu/icwdm_usdanwrc/2034 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. U.S. Department of Agriculture U.S. Government Publication Animal and Plant Health Inspection Service Wildlife Services Ecology of the Small 12 Indian Mongoose (Herpestes auropunctatus) in North America Are R. Berentsen, William C. Pitt, and Robert T. Sugihara CONTENTS General Ecology and Distribution......................................................................... -

Glimpse of an African… Wolf? Cécile Bloch
$6.95 Glimpse of an African… Wolf ? PAGE 4 Saving the Red Wolf Through Partnerships PAGE 9 Are Gray Wolves Still Endangered? PAGE 14 Make Your Home Howl Members Save 10% Order today at shop.wolf.org or call 1-800-ELY-WOLF Your purchases help support the mission of the International Wolf Center. VOLUME 25, NO. 1 THE QUARTERLY PUBLICATION OF THE INTERNATIONAL WOLF CENTER SPRING 2015 4 Cécile Bloch 9 Jeremy Hooper 14 Don Gossett In the Long Shadow of The Red Wolf Species Survival Are Gray Wolves Still the Pyramids and Beyond: Plan: Saving the Red Wolf Endangered? Glimpse of an African…Wolf? Through Partnerships In December a federal judge ruled Geneticists have found that some In 1967 the number of red wolves that protections be reinstated for of Africa’s golden jackals are was rapidly declining, forcing those gray wolves in the Great Lakes members of the gray wolf lineage. remaining to breed with the more wolf population area, reversing Biologists are now asking: how abundant coyote or not to breed at all. the USFWS’s 2011 delisting many golden jackals across Africa The rate of hybridization between the decision that allowed states to are a subspecies known as the two species left little time to prevent manage wolves and implement African wolf? Are Africa’s golden red wolf genes from being completely harvest programs for recreational jackals, in fact, wolves? absorbed into the expanding coyote purposes. If biological security is population. The Red Wolf Recovery by Cheryl Lyn Dybas apparently not enough rationale for Program, working with many other conservation of the species, then the organizations, has created awareness challenge arises to properly express and laid a foundation for the future to the ecological value of the species. -

Wild Animal, Birds and Reptiles Rescues from Unprotected Open Wells, Bore Wells and Water Tanks for Last Three Years by Animal Rahat
JOURNAL OF WILDLIFE RESEARCH Journal homepage: www.jakraya.com/journal/jwr STUDY REPORT Wild Animal, Birds and Reptiles Rescues from Unprotected Open Wells, Bore Wells and Water Tanks for Last Three Years by Animal Rahat 1* Chittora R.K., 2Upreti N.C., 3Jadhav A.S., 4Yadav C.D., 5Bhise P.R., 6Naik K.P. and 7Pol K.K. 1Senior Veterinary Trainer, 2Chief Operating Officer, 3Veterinary Field Officer, 4Clinical Quality Assurance Manager, 5Veterinary Field Officer, 6Community Facilitator and Animal Rescue Officer, 7Animal Welfare Inspector, Animal Rahat, Post Box No-30 Pin 416416, Maharashtra, India. Abstract The open wells, bore wells and water tanks (storage of water in big ponds on ground in advance system of agriculture) are the means of irrigation in most part of India but on the other hand these open wells, bore wells and water tanks are unprotected from top, while guidelines for *Corresponding Author: protecting of these structures are in the system. These unprotected open wells, bore wells and water tanks are nightmare for wild animals, they fall Chittora R. K. down into these structures accidentally when they are chased by other Email: [email protected] predator species or while playing or fighting with each other. Animal Rahat has rescued 43 wild animals, birds and reptiles including 10 species; namely Indian foxes, Jackals, Civets, Wolf, Indian spectacled cobra, Received: 11/05/2020 Russell’s viper, Indian rat snakes, Monitor lizard, Crocodile, Pea fowls Accepted: 25/05/2020 from these unprotected structures in last 3 years’ period i.e. from April 2017 to March 2020. Appropriate equipment’s, trained persons, and veterinarians along with rapid actions are necessary for rescue of these wild species without harming them as well as humans. -

Ecology of the European Badger (Meles Meles) in the Western Carpathian Mountains: a Review
Wildl. Biol. Pract., 2016 Aug 12(3): 36-50 doi:10.2461/wbp.2016.eb.4 REVIEW Ecology of the European Badger (Meles meles) in the Western Carpathian Mountains: A Review R.W. Mysłajek1,*, S. Nowak2, A. Rożen3, K. Kurek2, M. Figura2 & B. Jędrzejewska4 1 Institute of Genetics and Biotechnology, Faculty of Biology, University of Warsaw, Pawińskiego 5a, 02-106 Warszawa, Poland. 2 Association for Nature “Wolf”, Twardorzeczka 229, 34-324 Lipowa, Poland. 3 Institute of Environmental Sciences, Jagiellonian University, Gronostajowa 7, 30-387 Kraków, Poland. 4 Mammal Research Institute, Polish Academy of Sciences, Waszkiewicza 1c, 17-230 Białowieża, Poland. * Corresponding author email: [email protected]. Keywords Abstract Altitudinal Gradient; This article summarizes the results of studies on the ecology of the European Diet Composition; badger (Meles meles) conducted in the Western Carpathians (S Poland) Meles meles; from 2002 to 2010. Badgers inhabiting the Carpathians use excavated setts Mustelidae; (53%), caves and rock crevices (43%), and burrows under human-made Sett Utilization; constructions (4%) as permanent shelters. Excavated setts are located up Spatial Organization. to 640 m a.s.l., but shelters in caves and crevices can be found as high as 1,050 m a.s.l. Badger setts are mostly located on slopes with southern, eastern or western exposure. Within their territories, ranging from 3.35 to 8.45 km2 (MCP100%), badgers may possess 1-12 setts. Family groups are small (mean = 2.3 badgers), population density is low (2.2 badgers/10 km2), as is reproduction (0.57 young/year/10 km2). Hunting by humans is the main mortality factor (0.37 badger/year/10 km2). -
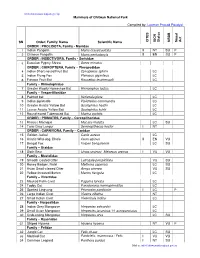
Mammals of Chitwan National Park Compiled By: Laxman Prasad Poudyal SN Order/ Family/ Name Scientific Name C IT E S IU C N S Ta
www.chitwannationalpark.gov.np Mammals of Chitwan National Park Compiled by: Laxman Prasad Poudyal SN Order/ Family/ Name Scientific Name CITES IUCN Status NRDB Nepal Act ORDER : PHOLIDOTA, Family - Manidae 1 Indian Pangolin Manis crassicaudata II NT SU P 2 Chinese Pangolin Manis pentadacyla II EN SU P ORDER : INSECTIVORA, Family - Soricidae 3 Eurasian Pygmy Shrew Sorex minutus ORDER : CHIROPTERA, Family – Pteropodidae 4 Indian Short-nosed Fruit Bat Cynopterus sphinx LC 5 Indian Flying Fox Pteropus giganteus LC 6 Fulvous Fruit Bat Rousettus leschenaulti LC Family – Rhinolophidae 7 Greater Woolly Horseshoe Bat Rhinolophus luctus LC Family – Vespertilionidae 8 Painted bat Kerivoula picta LC 9 Indian pipistrelle Pipistrellus coromandra LC 10 Greater Asiatic Yellow Bat Scotophilus heathi LC 11 Lesser Asiatic Yellow Bat Scotophilus kuhlii LC 12 Round-eared Tubenosed Bat Murina cyclotis LC ORDER : PRIMATES, Family – Cercopithecidae 13 Rhesus Macaque Macaca mulatta LC SU 14 Tarai Gray Langur Semnopithecus hector I NT ORDER : CARNIVORA, Family – Canidae 15 Golden Jackal Canis aureus LC 16 Asiatic Wild-dog, Dhole Cuon alpinus II EN VU 17 Bengal Fox Vulpes bengalensis LC SU Family – Ursidae 18 Sloth Bear Ursus ursinus/ Melursus ursinus I VU VU Family – Mustelidae 19 Smooth Coated Otter Lutrogale perspicillata VU SU 20 Honey Badger, Ratel Mellivora capensis LC SU 21 Asian Small-clawed Otter Aonyx cinerea VU SU 22 Yellow-throated Marten Martes flavigula LC Family – Viverridae 23 Masked Palm Civet Paguma larvata LC 24 Toddy Cat Paradoxurus hermaphroditus LC 25 Spotted Lingsang Prionodon pardicolor I LC P 26 Large Indian Civet Viverra zibetha NT 27 Small Indian Civet Viverricula indica LC Family - Herpestidae 28 Indian Grey Mongoose Herpestes edwardsii LC 29 Small Asian Mongoose Herpestes javanicus/ H. -

Asiatic Golden Cat in Thailand Population & Habitat Viability Assessment
Asiatic Golden Cat in Thailand Population & Habitat Viability Assessment Chonburi, Thailand 5 - 7 September 2005 FINAL REPORT Photos courtesy of Ron Tilson, Sumatran Tiger Conservation Program (golden cat) and Kathy Traylor-Holzer, CBSG (habitat). A contribution of the IUCN/SSC Conservation Breeding Specialist Group. Traylor-Holzer, K., D. Reed, L. Tumbelaka, N. Andayani, C. Yeong, D. Ngoprasert, and P. Duengkae (eds.). 2005. Asiatic Golden Cat in Thailand Population and Habitat Viability Assessment: Final Report. IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN. IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. © Copyright CBSG 2005 Additional copies of Asiatic Golden Cat of Thailand Population and Habitat Viability Assessment can be ordered through the IUCN/SSC Conservation Breeding Specialist Group, 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124, USA (www.cbsg.org). The CBSG Conservation Council These generous contributors make the work of CBSG possible Providers $50,000 and above Paignton Zoo Emporia Zoo Parco Natura Viva - Italy Laurie Bingaman Lackey Chicago Zoological Society Perth Zoo Lee Richardson Zoo -Chairman Sponsor Philadelphia Zoo Montgomery Zoo SeaWorld, Inc. -

Monitoring Amur Leopards and Tigers in Northeast China
Monitoring Amur Leopards and Tigers in Northeast China An Amur tiger investigates a camera trap in Jilin Province, China, May 2017. Photo © WCS China FINAL REPORT TO THE WILDCATS CONSERVATION ALLIANCE (WCCA) APRIL 2018 Grant Award: £18,746 Grant Period: January 1, 2017 - December 31, 2017 Report Period: January 1, 2017 - December 31, 2017 For more information please contact: Aimin Wang Libby Del Greco Director Senior Development Officer WCS China WCS Partners in Field Conservation Wildlife Conservation Society Wildlife Conservation Society Room 2-1101, Tower 2 2300 Southern Boulevard Ronghua Shijia, No. 29 Xiaoyingbeilu, Bronx, NY 10460 Chaoyang District, Beijing 100101 [email protected] [email protected] Tel: 718 741 1615 Tel: +86 010 84867735 Executive Summary The Wildlife Conservation Society’s (WCS) China Program has, for years, played an important role in conservation of Amur leopards and tigers in northeast China. In 2017, with support from the WildCats Conservation Alliance (WCCA), we continued our monitoring program by deploying 50 pairs of camera traps in Hunchun Nature Reserve (HNR) to cover approximately 450 km2 of key habitat for Amur tigers and leopards. The camera trap monitoring period covered about 25,000 trap nights and resulted in about 50,000 images and videos of wildlife and human activity, of which 700 images and videos were of 10 leopards and 13 tigers. Seven of these leopards and eight of these tigers were new to our camera traps. By combining data with partners collecting data across the entirety of the park, we identified 20 different leopard individuals (9 males, 5 females, and 6 of unknown sex), and 18 different tiger individuals (5 males, 5 females, and 8 of unknown sex) in HNR.