Notes on Mating Behaviour of Two Small Carnivores in Bangladesh
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphological Aspects of the Brain in the Indian Grey Mongoose (Herpestes Edwardsii)
Iranian Journal of Veterinary Received: 2020- Mar- 06 Accepted after revision: 2020- Nov- 01 Science and Technology Published online: 2021- Feb- 27 Short communication DOI: 10.22067/ijvst.2020.39237 Morphological aspects of the brain in the Indian grey mongoose (Herpestes Edwardsii) Babak Rasouli, Soghra Gholami, Younes Kamali Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran. ABSTRACT Mongoose is a common name for 29 to 34 species in 14 genera of the family Herpestidae which are found in vast areas of southwestern Asia, especially southern Iran. Anatomical and morphological studies of the brain have always been of interest to the researchers in the field of anatomy, due to its high importance in various fields of veterinary and zoology. Because of the lack of information about the brain structure in wild carnivores, the present study was conducted to better understand the morphological features in Indian grey mongoose. For this purpose, 4 carcasses of adult mongooses were used. They were found in different areas of Fars province. The mongooses had died due to natural causes. The brain was carefully separated from the skull and the measurements and observations were made on different parts of it. In this study, it wa found that the brain's structure has an ovoid appearance. Also, distinguished olfactory bulbs, deep transverse and longitudinal fissures, and relatively large cerebellar vermis were observed. Accord- ing to the current study, it can be concluded that the anatomical features of the brain in the mongoose are similar to those of other carnivores and are in perfect harmony with the sensory and motor capabilities of the animal. -

1. Biological Environment 1.1
EB Report for Expansion of Corporate Office Building, Noida (U.P.) ……….………………………………………………………………………………………………………………………………………………………………………………… 1. Biological Environment 1.1. Introduction Biodiversity reflects the potential of a regional ecosystem. Biota of a particular area is considered as indicators of the environment as they quickly respond not only to one environmental factor but also an interactive group of factors. These communities influence and react sensitively to changes in the balance of environmental stresses. Conservation of the biodiversity is essential for the sustainable development. Before starting any Environmental Impact Assessment study, it is necessary to identify the baseline of relevant environmental parameters which are likely to be affected as a result of the operation of the proposed project. A similar approach has been adopted for conducting the study on biological environment for this project. Both terrestrial and aquatic ecosystems have been studied to understand the biological environment nearby the project site. The study was conducted in the project area to assess all possible consequences on the biological environment. The present study is highlighting the various issues pertaining to floristic diversity and the faunal wealth in the core area i.e. Expansion of Corporate Office Building at Sector-16A, Film City, Noida (U.P.) and buffer zone i.e. area within 10 km radius. 1.1.1. Description of Study Area The present project proposes modification of the Expansion of Corporate Office Building which is located Sector-16A, Film City, Noida (U.P.) under the Seismic Zone –IV as per IS 1893 (Part I): 2002 (indicating high damage risk zone). The buildings will be designed as earthquake resistant and comply with IS specifications. -

The Yellow Mongoose Cynictis Penicillata in the Great Fish River Reserve (Eastern Cape, South Africa)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) Spatio-temporal ecology of the yellow mongoose Cynictis penicillata in the Great Fish River Reserve (Eastern Cape, South Africa) By Owen Akhona Mbatyoti A dissertation submitted in fulfilment of the requirements for the degree of MASTER OF SCIENCE (ZOOLOGY) in the Faculty of Science and Agriculture at the University of Fort Hare August 2012 Supervisor: Dr Emmanuel Do Linh San DECLARATION 1. This is to declare that this dissertation entitled “Spatio-temporal ecology of the yellow mongoose Cynictis penicillata in the Great Fish River Reserve (Eastern Cape, South Africa)” is my own work and has not been previously submitted to another institute. 2. I know that plagiarism means taking and using the ideas, writings, work or inventions of another person as if they were one’s own. I know that plagiarism not only includes verbatim copying, but also extensive use of another person’s ideas without proper acknowledgement (which sometimes includes the use of quotation marks). I know that plagiarism covers this sort of material found in textual sources (e.g. books, journal articles and scientific reports) and from the Internet. 3. I acknowledge and understand that plagiarism is wrong. 4. I understand that my research must be accurately referenced. I have followed the academic rules and conventions concerning referencing, citation and the use of quotations. 5. I have not allowed, nor will I in the future allow, anyone to copy my work with the intention of passing it off as their own work. -

Comparative Ecomorphology and Biogeography of Herpestidae and Viverridae (Carnivora) in Africa and Asia
Comparative Ecomorphology and Biogeography of Herpestidae and Viverridae (Carnivora) in Africa and Asia Gina D. Wesley-Hunt1, Reihaneh Dehghani2,3 and Lars Werdelin3 1Biology Department, Montgomery College, 51 Mannakee St. Rockville, Md. 20850, USA; 2Department of Zoology, Stockholm University, SE-106 91, Stockholm, Sweden; 3Department of Palaeozoology, Swedish Museum of Natural History, Box 50007, SE-104 05, Stockholm, Sweden INTRODUCTION Ecological morphology (ecomorphology) is a powerful tool for exploring diversity, ecology and evolution in concert (Wainwright, 1994, and references therein). Alpha taxonomy and diversity measures based on taxon counting are the most commonly used tools for understanding long-term evolutionary patterns and provide the foundation for all other biological studies above the organismal level. However, this provides insight into only a single dimension of a multidimensional system. As a complement, ecomorphology allows us to describe the diversification and evolution of organisms in terms of their morphology and ecological role. This is accomplished by using quantitative and semi-quantitative characterization of features of organisms that are important, for example, in niche partitioning or resource utilization. In this context, diversity is commonly referred to as disparity (Foote, 1993). The process of speciation, for example, can be better understood and hypotheses more rigorously tested, if it can be quantitatively demonstrated whether a new species looks very similar to the original taxon or whether its morphology has changed in a specific direction. For example, if a new species of herbivore evolves with increased grinding area in the cheek dentition, it can either occupy the same area of morphospace as previously existing species, suggesting increased resource competition, or it can occupy an area of morphospace that had previously been empty, suggesting evolution into a new niche. -

Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal
SMALL CARNIVORES IN TINJURE-MILKE-JALJALE, EASTERN NEPAL The content of this booklet can be used freely with permission for any conservation and education purpose. However we would be extremely happy to get a hard copy or soft copy of the document you have used it for. For further information: Friends of Nature Kathmandu, Nepal P.O. Box: 23491 Email: [email protected], Website: www.fonnepal.org Facebook: www.facebook.com/fonnepal2005 First Published: April, 2018 Photographs: Friends of Nature (FON), Jeevan Rai, Zaharil Dzulkafly, www.pixabay/ werner22brigitte Design: Roshan Bhandari Financial support: Rufford Small Grants, UK Authors: Jeevan Rai, Kaushal Yadav, Yadav Ghimirey, Som GC, Raju Acharya, Kamal Thapa, Laxman Prasad Poudyal and Nitesh Singh ISBN: 978-9937-0-4059-4 Acknowledgements: We are grateful to Zaharil Dzulkafly for his photographs of Marbled Cat, and Andrew Hamilton and Wildscreen for helping us get them. We are grateful to www.pixabay/werner22brigitte for giving us Binturong’s photograph. We thank Bidhan Adhikary, Thomas Robertson, and Humayra Mahmud for reviewing and providing their valuable suggestions. Preferred Citation: Rai, J., Yadav, K., Ghimirey, Y., GC, S., Acharya, R., Thapa, K., Poudyal, L.P., and Singh, N. 2018. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal. Friends of Nature, Nepal and Rufford Small Grants, UK. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal Why Protect Small Carnivores! Small carnivores are an integral part of our ecosystem. Except for a few charismatic species such as Red Panda, a general lack of research and conservation has created an information gap about them. I am optimistic that this booklet will, in a small way, be the starting journey of filling these gaps in our knowledge bank of small carnivore in Nepal. -

Ecology of the Small Indian Mongoose (Herpestes Auropunctatus) in North America
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Publications Plant Health Inspection Service 2018 Ecology of the Small Indian Mongoose (Herpestes auropunctatus) in North America Are R. Berentsen USDA National Wildlife Research Center, [email protected] William C. Pitt Smithsonian Institute Robert T. Sugihara USDA/APHIS/WS/National Wildlife Research Center Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Part of the Life Sciences Commons Berentsen, Are R.; Pitt, William C.; and Sugihara, Robert T., "Ecology of the Small Indian Mongoose (Herpestes auropunctatus) in North America" (2018). USDA National Wildlife Research Center - Staff Publications. 2034. https://digitalcommons.unl.edu/icwdm_usdanwrc/2034 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. U.S. Department of Agriculture U.S. Government Publication Animal and Plant Health Inspection Service Wildlife Services Ecology of the Small 12 Indian Mongoose (Herpestes auropunctatus) in North America Are R. Berentsen, William C. Pitt, and Robert T. Sugihara CONTENTS General Ecology and Distribution......................................................................... -
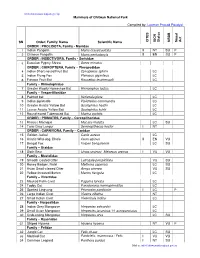
Mammals of Chitwan National Park Compiled By: Laxman Prasad Poudyal SN Order/ Family/ Name Scientific Name C IT E S IU C N S Ta
www.chitwannationalpark.gov.np Mammals of Chitwan National Park Compiled by: Laxman Prasad Poudyal SN Order/ Family/ Name Scientific Name CITES IUCN Status NRDB Nepal Act ORDER : PHOLIDOTA, Family - Manidae 1 Indian Pangolin Manis crassicaudata II NT SU P 2 Chinese Pangolin Manis pentadacyla II EN SU P ORDER : INSECTIVORA, Family - Soricidae 3 Eurasian Pygmy Shrew Sorex minutus ORDER : CHIROPTERA, Family – Pteropodidae 4 Indian Short-nosed Fruit Bat Cynopterus sphinx LC 5 Indian Flying Fox Pteropus giganteus LC 6 Fulvous Fruit Bat Rousettus leschenaulti LC Family – Rhinolophidae 7 Greater Woolly Horseshoe Bat Rhinolophus luctus LC Family – Vespertilionidae 8 Painted bat Kerivoula picta LC 9 Indian pipistrelle Pipistrellus coromandra LC 10 Greater Asiatic Yellow Bat Scotophilus heathi LC 11 Lesser Asiatic Yellow Bat Scotophilus kuhlii LC 12 Round-eared Tubenosed Bat Murina cyclotis LC ORDER : PRIMATES, Family – Cercopithecidae 13 Rhesus Macaque Macaca mulatta LC SU 14 Tarai Gray Langur Semnopithecus hector I NT ORDER : CARNIVORA, Family – Canidae 15 Golden Jackal Canis aureus LC 16 Asiatic Wild-dog, Dhole Cuon alpinus II EN VU 17 Bengal Fox Vulpes bengalensis LC SU Family – Ursidae 18 Sloth Bear Ursus ursinus/ Melursus ursinus I VU VU Family – Mustelidae 19 Smooth Coated Otter Lutrogale perspicillata VU SU 20 Honey Badger, Ratel Mellivora capensis LC SU 21 Asian Small-clawed Otter Aonyx cinerea VU SU 22 Yellow-throated Marten Martes flavigula LC Family – Viverridae 23 Masked Palm Civet Paguma larvata LC 24 Toddy Cat Paradoxurus hermaphroditus LC 25 Spotted Lingsang Prionodon pardicolor I LC P 26 Large Indian Civet Viverra zibetha NT 27 Small Indian Civet Viverricula indica LC Family - Herpestidae 28 Indian Grey Mongoose Herpestes edwardsii LC 29 Small Asian Mongoose Herpestes javanicus/ H. -

The 2008 IUCN Red Listings of the World's Small Carnivores
The 2008 IUCN red listings of the world’s small carnivores Jan SCHIPPER¹*, Michael HOFFMANN¹, J. W. DUCKWORTH² and James CONROY³ Abstract The global conservation status of all the world’s mammals was assessed for the 2008 IUCN Red List. Of the 165 species of small carni- vores recognised during the process, two are Extinct (EX), one is Critically Endangered (CR), ten are Endangered (EN), 22 Vulnerable (VU), ten Near Threatened (NT), 15 Data Deficient (DD) and 105 Least Concern. Thus, 22% of the species for which a category was assigned other than DD were assessed as threatened (i.e. CR, EN or VU), as against 25% for mammals as a whole. Among otters, seven (58%) of the 12 species for which a category was assigned were identified as threatened. This reflects their attachment to rivers and other waterbodies, and heavy trade-driven hunting. The IUCN Red List species accounts are living documents to be updated annually, and further information to refine listings is welcome. Keywords: conservation status, Critically Endangered, Data Deficient, Endangered, Extinct, global threat listing, Least Concern, Near Threatened, Vulnerable Introduction dae (skunks and stink-badgers; 12), Mustelidae (weasels, martens, otters, badgers and allies; 59), Nandiniidae (African Palm-civet The IUCN Red List of Threatened Species is the most authorita- Nandinia binotata; one), Prionodontidae ([Asian] linsangs; two), tive resource currently available on the conservation status of the Procyonidae (raccoons, coatis and allies; 14), and Viverridae (civ- world’s biodiversity. In recent years, the overall number of spe- ets, including oyans [= ‘African linsangs’]; 33). The data reported cies included on the IUCN Red List has grown rapidly, largely as on herein are freely and publicly available via the 2008 IUCN Red a result of ongoing global assessment initiatives that have helped List website (www.iucnredlist.org/mammals). -

Pest Risk Assessment
PEST RISK ASSESSMENT Meerkat Suricata suricatta (Photo: Fir0002. Image from Wikimedia Commons under a GNU Free Documentation License, Version 1.2) March 2011 Department of Primary Industries, Parks, Water and Environment Resource Management and Conservation Division Department of Primary Industries, Parks, Water and Environment 2011 Information in this publication may be reproduced provided that any extracts are acknowledged. This publication should be cited as: DPIPWE (2011) Pest Risk Assessment: Meerkat (Suricata suricatta). Department of Primary Industries, Parks, Water and Environment. Hobart, Tasmania. About this Pest Risk Assessment This pest risk assessment is developed in accordance with the Policy and Procedures for the Import, Movement and Keeping of Vertebrate Wildlife in Tasmania (DPIPWE 2011). The policy and procedures set out conditions and restrictions for the importation of controlled animals pursuant to s32 of the Nature Conservation Act 2002. This pest risk assessment is prepared by DPIPWE for the use within the Department. For more information about this Pest Risk Assessment, please contact: Wildlife Management Branch Department of Primary Industries, Parks, Water and Environment Address: GPO Box 44, Hobart, TAS. 7001, Australia. Phone: 1300 386 550 Email: [email protected] Visit: www.dpipwe.tas.gov.au Disclaimer The information provided in this Pest Risk Assessment is provided in good faith. The Crown, its officers, employees and agents do not accept liability however arising, including liability for negligence, for any loss resulting from the use of or reliance upon the information in this Pest Risk Assessment and/or reliance on its availability at any time. Pest Risk Assessment: Meerkat Suricata suricatta 2/22 1. -

Herpestes Ichneumon, Egyptian Mongoose
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T41613A45207211 Herpestes ichneumon, Egyptian Mongoose Assessment by: Do Linh San, E., Maddock, A.H., Gaubert, P. & Palomares, F. View on www.iucnredlist.org Citation: Do Linh San, E., Maddock, A.H., Gaubert, P. & Palomares, F. 2016. Herpestes ichneumon. The IUCN Red List of Threatened Species 2016: e.T41613A45207211. http://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T41613A45207211.en Copyright: © 2016 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the -

Web Ecology 7: 53–62
Web Ecology 7: 53–62. Trophic niche partitioning between two native and two exotic carnivores in SW Portugal Maria João Santos, Bruno Miguel Pinto and Margarida Santos-Reis Santos, M. J., Pinto, B. M. and Santos-Reis, M. Trophic niche partitioning between two native and two exotic carnivores in SW Portugal. – Web Ecol. 7: 53–62. The introduction of exotic species is one of the most pervasive consequences of the increased human mobility. The most known negative effects are the decrease or extinc- tion of natives. The common-genet, Genetta genetta, and the Egyptian mongoose, Her- pestes ichneumon, were introduced in the Iberian Peninsula in the 15th and 19th centu- ries, respectively. The competitive exclusion principle defines that two ecologically simi- lar species cannot coexist. Thus, some degree of partitioning has to occur in species realized niche, which can occur at the trophic level. To test this hypothesis of partitio- ning we compared the diet of these two exotic species with that of two native species (stone marten, Martes foina, and red fox, Vulpes vulpes). The results show a high degree of overlap (>45%) between the diets of species similar in their feeding strategies (arbore- al and ground feeding). Nonetheless, at the finer scale of prey consumed at the species level some differences are found between the native and exotic species. These results suggest that if coexistence is due to trophic niche partitioning it only occurs at the level of the consumed species. However, coexistence may also be due to a combination of different strategies (home-range size, time and space use) that structured the different realized niches of each species. -

Small Carnivores
SMALL CARNIVORES IN TINJURE-MILKE-JALJALE, EASTERN NEPAL The content of this booklet can be used freely with permission for any conservation and education purpose. However we would be extremely happy to get a hard copy or soft copy of the document you have used it for. For further information: Friends of Nature Kathmandu, Nepal P.O. Box: 23491 Email: [email protected], Website: www.fonnepal.org Facebook: www.facebook.com/fonnepal2005 First Published: April, 2018 Photographs: Friends of Nature (FON), Jeevan Rai, Zaharil Dzulkafly, www.pixabay/ werner22brigitte Design: Roshan Bhandari Financial support: Rufford Small Grants, UK Authors: Jeevan Rai, Kaushal Yadav, Yadav Ghimirey, Som GC, Raju Acharya, Kamal Thapa, Laxman Prasad Poudyal and Nitesh Singh ISBN: 978-9937-0-4059-4 Acknowledgements: We are grateful to Zaharil Dzulkafly for his photographs of Marbled Cat, and Andrew Hamilton and Wildscreen for helping us get them. We are grateful to www.pixabay/werner22brigitte for giving us Binturong’s photograph. We thank Bidhan Adhikary, Thomas Robertson, and Humayra Mahmud for reviewing and providing their valuable suggestions. Preferred Citation: Rai, J., Yadav, K., Ghimirey, Y., GC, S., Acharya, R., Thapa, K., Poudyal, L.P., and Singh, N. 2018. Small Carnivores in Tinjure-Milke -Jaljale, Eastern Nepal. Friends of Nature, Nepal and Rufford Small Grants, UK. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal Why Protect Small Carnivore! Small carnivores are an integral part of our ecosystem. Except for a few charismatic species such as Red Panda, a general lack of research and conservation has created an information gap about them. I am optimistic that this booklet will, in a small way, be the starting journey of filling these gaps in our knowledge bank of small carnivore in Nepal.