Hengrui Medicine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Service Summary
COSCO SHIPPING TRANSPACIFIC SERVICE OVERVIEW Service Summary 23 SERVICE LINES cover 161 PORT PAIRS since 1st. April, 2017 * Including COSCO SHIPPING Out-Alliance Service Lines PSW/PNW/AWE SERVICE LINE OVERVIEW PSW Service Summary 12 Far-east to Southwest Coast of America Service lines Cover 61 Port Pairs CEN (COSCO)* AAC (COSCO) AAC2 (CMA+EMC) AAC3 (COSCO+WHL+PIL)** AAC4 (OOCL) Service 6 X 10000 6 X 10000 6 X 9000 6 X 8500 5 X 7800 Port ETB Port ETB Port ETB Port ETB Port ETB XINGANG 0 DALIAN 0 QINGDAO 0 QINGDAO 0 NINGBO 0 QINGDAO 3 LIANYUNGANG 1 SHANGHAI 2 SHANGHAI 2 SHANGHAI 1 SHANGHAI 5 SHANGHAI 4 NINGBO 4 NINGBO 3 PUSAN 4 Port Load of Port NINGBO 6 PRINCE RUPERT 17 LONG BEACH 20 LONG BEACH 18 LONG BEACH 17 LONG BEACH 16 LONG BEACH 22 SEATTLE 28 OAKLAND 23 OAKLAND 22 PUSAN 33 OAKLAND 27 DALIAN 42 TOKYO 38 QINGDAO 42 NINGBO 35 XINGANG 42 NAGOYA 39 QINGDAO 42 Port Discharge of Port *The details of all the services will be optimized further. ** COSCO SHIPPING’s Out-Alliance Service Lines PSW Service Summary 12 Far-east to Southwest Coast of America Service lines Cover 61 Port Pairs AAS (OOCL) AAS2 (CMA) AAS3 (EMC) AAS4 (EMC) Service 6 X 9000 6 X 14000 6 X 6500 6 X 7000 Port ETB Port ETB Port ETB Port ETB CAI MEP 0 FUQING 0 TAIPEI 0 YANTIAN 0 SHEKOU 3 NANSHA 2 XIAMEN 3 HONG KONG 1 HONG KONG 3 HONG KONG 3 SHEKOU 4 KAOHSIUNG 3 Port of Load Port YANTIAN 4 YANTIAN 4 YANTIAN 5 TAIPEI 4 KAOHSIUNG 6 XIAMEN 6 LONG BEACH 19 LONG BEACH 20 LONG BEACH 20 LONG BEACH 18 KAOHSIUNG 38 OAKLAND 25 OAKLAND 24 OAKLAND 22 CAI MEP 42 FUQING 42 TAIPEI 42 -

Appendix 1: Rank of China's 338 Prefecture-Level Cities
Appendix 1: Rank of China’s 338 Prefecture-Level Cities © The Author(s) 2018 149 Y. Zheng, K. Deng, State Failure and Distorted Urbanisation in Post-Mao’s China, 1993–2012, Palgrave Studies in Economic History, https://doi.org/10.1007/978-3-319-92168-6 150 First-tier cities (4) Beijing Shanghai Guangzhou Shenzhen First-tier cities-to-be (15) Chengdu Hangzhou Wuhan Nanjing Chongqing Tianjin Suzhou苏州 Appendix Rank 1: of China’s 338 Prefecture-Level Cities Xi’an Changsha Shenyang Qingdao Zhengzhou Dalian Dongguan Ningbo Second-tier cities (30) Xiamen Fuzhou福州 Wuxi Hefei Kunming Harbin Jinan Foshan Changchun Wenzhou Shijiazhuang Nanning Changzhou Quanzhou Nanchang Guiyang Taiyuan Jinhua Zhuhai Huizhou Xuzhou Yantai Jiaxing Nantong Urumqi Shaoxing Zhongshan Taizhou Lanzhou Haikou Third-tier cities (70) Weifang Baoding Zhenjiang Yangzhou Guilin Tangshan Sanya Huhehot Langfang Luoyang Weihai Yangcheng Linyi Jiangmen Taizhou Zhangzhou Handan Jining Wuhu Zibo Yinchuan Liuzhou Mianyang Zhanjiang Anshan Huzhou Shantou Nanping Ganzhou Daqing Yichang Baotou Xianyang Qinhuangdao Lianyungang Zhuzhou Putian Jilin Huai’an Zhaoqing Ningde Hengyang Dandong Lijiang Jieyang Sanming Zhoushan Xiaogan Qiqihar Jiujiang Longyan Cangzhou Fushun Xiangyang Shangrao Yingkou Bengbu Lishui Yueyang Qingyuan Jingzhou Taian Quzhou Panjin Dongying Nanyang Ma’anshan Nanchong Xining Yanbian prefecture Fourth-tier cities (90) Leshan Xiangtan Zunyi Suqian Xinxiang Xinyang Chuzhou Jinzhou Chaozhou Huanggang Kaifeng Deyang Dezhou Meizhou Ordos Xingtai Maoming Jingdezhen Shaoguan -

Association Between Blood Glucose Levels in Insulin Therapy And
Association between blood glucose levels in insulin therapy and Glasgow Outcome Score in patients with traumatic brain injury: secondary analysis of a randomized trial Tao Yuan Department of Neurosurgery, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Hongyu He Department of Neurosurgery, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Yuepeng Liu Center for clinical research and translational medicine, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Jianwei Wang Department of Neurosurgery, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Xin Kang Department of Neurosurgery, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Guanghui Fu Department of Neurosurgery, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Fangfang Xie Department of Neurosurgery, the aliated Lianyungang Oriental Hospital of Xuzhou Medical University Aimin Li Lianyungang No 1 People's Hospital Jun Chen Lianyungang No 1 People's Hospital Wen-xue Wang ( [email protected] ) Department of Neurosurgery,the aliated Lianyungang Oriental Hospital of Xuzhou Medical University https://orcid.org/0000-0002-9865-6811 Research Article Keywords: Traumatic brain injuries, Glasgow Outcome Score, hyperglycaemia, insulin therapy Posted Date: June 14th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-615839/v1 Page 1/18 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 2/18 Abstract Background: Too high or low blood glucose levels after traumatic brain injury (TBI) negatively affect the prognosis of patients with TBI. This study aimed to examine the relationship between different levels of blood glucose in insulin therapy and Glasgow Outcome Score (GOS) in patients with TBI. -

Effects of an Innovative Training Program for New Graduate Registered Nurses: A
medRxiv preprint doi: https://doi.org/10.1101/2020.09.11.20192468; this version posted September 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Effects of an Innovative Training Program for New Graduate Registered Nurses: a Comparison Study Fengqin Xu 1*, Yinhe Wang 2*, Liang Ma 1, Jiang Yu 1, Dandan Li 1, Guohui Zhou 1, Yuzi Xu 3, Hailin Zhang 1, Yang Cao 4 1 The First Affiliated Hospital of Kangda College of Nanjing Medical University, the First People’s Hospital of Lianyungang, the Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang 222000, Jiangsu, China 2 Department of Orthopaedic Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, 210008, Jiangsu, China 3 Zhejiang University School of Medicine, Hangzhou 310058, Zhejiang, China 4 Clinical Epidemiology and Biostatistics, School of Medical Sciences, Örebro University, Örebro 70182, Sweden * The authors contributed equally to this work. Correspondence authors: Yang Cao, Clinical Epidemiology and Biostatistics, School of Medical Sciences, Örebro University, Örebro 70182, Sweden. Email: [email protected] Hailin Zhang, the First Affiliated Hospital of Kangda College of Nanjing Medical University, the First People’s Hospital of Lianyungang, the Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang 222000, Jiangsu, China. 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Lung Transplantation As Therapeutic Option in Acute Respiratory Distress Syndrome for Coronavirus Disease 2019-Related Pulmonary fibrosis
Original Article Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis Jing-Yu Chen1, Kun Qiao2, Feng Liu1,BoWu1, Xin Xu3, Guo-Qing Jiao4, Rong-Guo Lu1, Hui-Xing Li1, Jin Zhao1, Jian Huang1, Yi Yang5, Xiao-Jie Lu6, Jia-Shu Li7, Shu-Yun Jiang8, Da-Peng Wang8, Chun-Xiao Hu9, Gui-Long Wang9, Dong-Xiao Huang9, Guo-Hui Jiao1, Dong Wei1, Shu-Gao Ye1, Jian-An Huang10, Li Zhou1, Xiao-Qin Zhang1, Jian-Xing He3 1Wuxi Lung Transplant Center, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, Jiangsu 214023, China; 2Department of Thoracic Surgery, Shenzhen Third People’s Hospital, Shenzhen, Guangdong 518100, China; 3Department of Thoracic Surgery/Oncology, State Key Laboratory and National Clinical Research Center for Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong 510120, China; 4Department of Cardiothoracic Surgery, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, Jiangsu 214023, China; 5Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu 210009, China; 6Wuxi Fifth Hospital, Wuxi, Jiangsu 214000, China; 7Department of Respiratory Medicine and Critical Care Medicine, The First People’s Hospital of Lianyungang City, Lianyungang, Jiangsu 222061, China; 8Department of Critical Care Medicine, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, Jiangsu 214023, China; 9Department of Anesthesiology, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, Jiangsu 214023, China; 10Department of Respiratory Medicine, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu 215006, China. Abstract Background: Critical patients with the coronavirus disease 2019 (COVID-19), even those whose nucleic acid test results had turned negative and those receiving maximal medical support, have been noted to progress to irreversible fatal respiratory failure. -

Jiangsu(PDF/288KB)
Mizuho Bank China Business Promotion Division Jiangsu Province Overview Abbreviated Name Su Provincial Capital Nanjing Administrative 13 cities and 45 counties Divisions Secretary of the Luo Zhijun; Provincial Party Li Xueyong Committee; Mayor 2 Size 102,600 km Shandong Annual Mean 16.2°C Jiangsu Temperature Anhui Shanghai Annual Precipitation 861.9 mm Zhejiang Official Government www.jiangsu.gov.cn URL Note: Personnel information as of September 2014 [Economic Scale] Unit 2012 2013 National Share (%) Ranking Gross Domestic Product (GDP) 100 Million RMB 54,058 59,162 2 10.4 Per Capita GDP RMB 68,347 74,607 4 - Value-added Industrial Output (enterprises above a designated 100 Million RMB N.A. N.A. N.A. N.A. size) Agriculture, Forestry and Fishery 100 Million RMB 5,809 6,158 3 6.3 Output Total Investment in Fixed Assets 100 Million RMB 30,854 36,373 2 8.2 Fiscal Revenue 100 Million RMB 5,861 6,568 2 5.1 Fiscal Expenditure 100 Million RMB 7,028 7,798 2 5.6 Total Retail Sales of Consumer 100 Million RMB 18,331 20,797 3 8.7 Goods Foreign Currency Revenue from Million USD 6,300 2,380 10 4.6 Inbound Tourism Export Value Million USD 328,524 328,857 2 14.9 Import Value Million USD 219,438 221,987 4 11.4 Export Surplus Million USD 109,086 106,870 3 16.3 Total Import and Export Value Million USD 547,961 550,844 2 13.2 Foreign Direct Investment No. of contracts 4,156 3,453 N.A. -

The Development of the City with the Historical District: the Comparison with Suzhou and Nantong
The Development of the City with the Historical District: The Comparison with Suzhou and Nantong Shan Lu, Southeast University, China The Asian Conference on Asian Studies 2019 Official Conference Proceedings Abstract The current construction of some historical district in China has become a social hot issue. On the one hand, the historical district as a space carrier with a high concentration of regional natural environment, history and culture, urban construction and other elements has high value for protecting the historical heritage of the city and highlighting the urban characteristics. On the other hand, driven by the huge land value and economic value, along with the rapid development of the city, the historical district suffer a considerable degree of constructive damage and is difficult to recover. Therefore, a comprehensive analysis of the contradiction between ancient city protection and urban development, and achieving a win-win situation between urban development and historical district protection is a key technical issue in contemporary urban design. This article compares and analyzes the case of Suzhou and Nantong and uses historical mapping and research interview method to analyze the relationship between historical district protection and urban development. First of all, it analyzes the urban development status of the two cities. Secondly, five key issues are identified: urban pattern change, regional function renewal, infrastructure optimization, spatial shape adjustment and lifestyle change. Then analyze its main constraints from three aspects of economy, policy and design. Finally, five strategies are proposed to explore the future development of modern city and historical district protection: Dislocation development, Featured positioning, Regional service, Morphological style and Flexible adjustment. -

Best-Performing Cities: China 2018
Best-Performing Cities CHINA 2018 THE NATION’S MOST SUCCESSFUL ECONOMIES Michael C.Y. Lin and Perry Wong MILKEN INSTITUTE | BEST-PERFORMING CITIES CHINA 2018 | 1 Acknowledgments The authors are grateful to Laura Deal Lacey, executive director of the Milken Institute Asia Center, Belinda Chng, the center’s director for policy and programs, and Ann-Marie Eu, the Institute’s senior associate for communications, for their support in developing this edition of our Best- Performing Cities series focused on China. We thank the communications team for their support in publication as well as Kevin Klowden, the executive director of the Institute’s Center for Regional Economics, Minoli Ratnatunga, director of regional economic research at the Institute, and our colleagues Jessica Jackson and Joe Lee for their constructive comments on our research. About the Milken Institute We are a nonprofit, nonpartisan think tank determined to increase global prosperity by advancing collaborative solutions that widen access to capital, create jobs, and improve health. We do this through independent, data-driven research, action-oriented meetings, and meaningful policy initiatives. About the Asia Center The Milken Institute Asia Center promotes the growth of inclusive and sustainable financial markets in Asia by addressing the region’s defining forces, developing collaborative solutions, and identifying strategic opportunities for the deployment of public, private, and philanthropic capital. Our research analyzes the demographic trends, trade relationships, and capital flows that will define the region’s future. About the Center for Regional Economics The Center for Regional Economics promotes prosperity and sustainable growth by increasing understanding of the dynamics that drive job creation and promote industry expansion. -
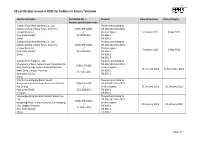
CE Certificates Issued in 2021 for Holders in China / Vietnam
CE certificates issued in 2021 for holders in China / Vietnam Certificate holder Certificate No. / Product Date of issuance Date of expiry Product specification code Xuzhou Yizun New Material Co., Ltd. Plywood according to Industrial Park, Hegou Town, Xinyi City, 0766-CPR-429/4 EN 636:2012+A1:2015 Jiangsu Province / Product types: 5 January 2021 9 May 2021 Post code 221439 2117181-009 EN 636-1 China EN 636-2 Xuzhou Yizun New Material Co., Ltd. Plywood according to Industrial Park, Hegou Town, Xinyi City, 0766-CPR-430/4 EN 636:2012+A1:2015 Jiangsu Province / Product types: 5 January 2021 9 May 2021 Post code 221439 2117181-010 EN 636-1 China EN 636-2 EN 636-3 Xuzhou Kinri Trade Co., Ltd. Plywood according to Shengyang Village, Sanpu Town, Tongshan Dis- EN 636:2012+A1:2015 0766-CPR-564 trict, Xuzhou High-tech Industrial Develop- Product types: / 11 January 2021 24 November 2021 ment Zone, Jiangsu Province EN 636-1 2121007-005 Post code 221112 EN 636-2 China Viet Nam Hai Duong Baifar Wood Plywood according to Nam Sach Industrial Zone, Nam Sach District, 0766-CPR-565 EN 636:2012+A1:2015 Hai Duong / Product types: 11 January 2021 10 January 2022 Post code 03000 2121008-001 EN 636-1 Vietnam EN 636-2 LianyungangChanta International Wood Cov Plywood according to Ltd. EN 636:2012+A1:2015 0766-CPR-485/2 Kangpeng Plaza, Lianyun District, Lianyungang Product types: / 26 January 2021 20 January 2022 City, Jiangsu Province EN 636-1 2119007-003 Post code 222000 EN 636-2 China EN 636-3 Page 1 of 7 CE certificates issued in 2021 for holders in China / Vietnam Certificate holder Certificate No. -

SUZHOU Suzhou Asian City Report Suzhou Asian City Report
– 1H/2020 Asian City Report 25th Floor, Two ICC MARKET No. 288 South Shaanxi Road IN Shanghai MINUTES China Savills Research SUZHOU Suzhou Asian City Report Suzhou Asian City Report Grade A office rents fall as vacancy rates reach multi- COVID-19 had a significant impact on Suzhou’s retail year highs market, both for landlords and tenants SUPPLY AND DEMAND SUPPLY AND DEMAND GRAPH 3: Shopping Mall Supply and Vacancy Rate, 2015 to GRAPH 1: Suzhou Grade A Office Market New Supply, Net Take-up Suzhou’s retail sales contracted by 9.2% YoY in the first six months of 2020 and Vacancy Rate, 2015 to 1H/2020 Suzhou has seen a swift economic recovery following the outbreak of 1H/2020 Supply (LHS) Take-up (LHS) Vacancy (RHS) COVID-19 and the subsequent lockdowns. Industrial added value from due to the continued fallout from COVID-19, though this figure compares favourably with the 18.4% contraction recorded in the first quarter. Online 600,000 35% above a designated scale in Suzhou increased by 1.6% year-on-year (YoY) Supply (LHS) Vacancy (RHS) in 1H/2020, while the value of monthly output recorded four consecutive retail sales, however, recorded phenomenal growth, expanding 37.6% 1,250,000 15% months of growth. Suzhou’s four leading industries, namely biomedicine, YoY. Under the promotion of policies such as the “night economy” and 500,000 30% new-generation information technology, nanotechnology and artificial consumer coupons, Suzhou’s retail market recovered swiftly in 1H/2020. intelligence contributed 21.6% of total added value. -

Time to Sputum Culture Conversion and Treatment Outcome of Patients with Multidrug-Resistant Tuberculosis: a Prospective Cohort Study from Urban China
AGORA | | RESEARCH LETTER Time to sputum culture conversion and treatment outcome of patients with multidrug-resistant tuberculosis: a prospective cohort study from urban China To the Editor: Sputum culture plays an important role in monitoring treatment response in patients with multidrug-resistant tuberculosis (MDR-TB), and sputum culture conversion is a clinical tool used to predict therapeutic efficacy [1]. Monthly culture monitoring is essential for earlier detection of treatment failure in patients with MDR-TB. More sensitive signals of nonresponse would further avoid adverse outcomes [2]. Evidence exists suggesting that sputum culture conversion after 2 months of treatment may be an early predictor of treatment success in patients with MDR-TB [3, 4]. Although sputum culture conversion has been used substantially in clinical settings, few prospective cohort studies have investigated its efficacy after different durations of MDR-TB treatment, and no studies have been performed in China or India [5, 6]. We evaluated whether 2-, 3-, 6- or 24-month culture conversion predicted treatment success in patients with MDR-TB. The study was conducted in four cities (Xuzhou, Lianyungang, Zhenjiang and Nantong) in Jiangsu province, China. Xuzhou and Lianyungang, both located in the north of Jiangsu province, are economically underdeveloped areas with a higher TB burden while Zhenjiang, situated in the south, is a relatively rich city with a lighter TB burden, and Nantong, located in the middle of Jiangsu, is a middle-income developed city with a moderate TB burden [7]. All patients with MDR-TB were enrolled consecutively between December 2011 and March 2014. Patients with MDR-TB were identified at the time of diagnosis by regional reference laboratories using traditional drug sensitivity tests (DST), as previously described [8]. -

Federal Register/Vol. 86, No. 12/Thursday, January 21, 2021
6300 Federal Register / Vol. 86, No. 12 / Thursday, January 21, 2021 / Notices Dated: January 12, 2021. 9. Linyi Huasheng Yongbin Wood Co., Ltd. International Trade Administration, Jeffrey I. Kessler, 10. Linyi Jiahe Wood Industry Co., Ltd. U.S. Department of Commerce, 1401 11. Linyi Sanfortune Wood Co., Ltd. Assistant Secretary for Enforcement and Constitution Avenue NW, Washington, 12. Qingdao Top P&Q International Corp. Compliance. DC 20230; telephone: (202) 482–5305. 13. Shandong Qishan International Trading Appendix I Co., Ltd. SUPPLEMENTARY INFORMATION: 14. Shanghai Brightwood Trading Co., Ltd. Background Companies Not Eligible for a Separate Rate 15. Shanghai Futuwood Trading Co., Ltd. 1. Feixian Longteng Wood Co., Ltd. 16. Shanghai Luli Trading Co., Ltd. On July 28, 2020, Commerce 2. Golder International Trade Co., Ltd. 17. Suining Pengxiang Wood Co., Ltd. published the preliminary results of this 3. Highland Industries-Hanlin 18. Suqian Hopeway International Trade Co., administrative review.1 We invited 4. Huainan Mengping Import and Export Co., Ltd. parties to comment on the Preliminary Ltd. 19. Suzhou Oriental Dragon Import and Results. No party submitted comments. 5. Jiangsu High Hope Arser Co., Ltd.27 Export Co., Ltd. 6. Jiangsu Sunwell Cabinetry Co., Ltd. 20. Xuzhou Jiangheng Wood Products Co., Accordingly, the final results remain 7. Jiangsu Top Point International Co., Ltd. Ltd. unchanged from the Preliminary 8. Jiaxing Gsun Imp. & Exp. Co., Ltd. 21. Xuzhou Jiangyang Wood Industries Co., Results. Ltd. 9. Lianyungang Yuantai International Trade Scope of the Order Co., Ltd. 22. Xuzhou Timber International Trade Co., 10. Linyi Bomei Furniture Co., Ltd. Ltd. The scope of the order covers 11.