Constraints on the Composition of Trojan Asteroid 624 Hektor Dale P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Occultation Evidence for a Satellite of the Trojan Asteroid (911) Agamemnon Bradley Timerson1, John Brooks2, Steven Conard3, David W
Occultation Evidence for a Satellite of the Trojan Asteroid (911) Agamemnon Bradley Timerson1, John Brooks2, Steven Conard3, David W. Dunham4, David Herald5, Alin Tolea6, Franck Marchis7 1. International Occultation Timing Association (IOTA), 623 Bell Rd., Newark, NY, USA, [email protected] 2. IOTA, Stephens City, VA, USA, [email protected] 3. IOTA, Gamber, MD, USA, [email protected] 4. IOTA, KinetX, Inc., and Moscow Institute of Electronics and Mathematics of Higher School of Economics, per. Trekhsvyatitelskiy B., dom 3, 109028, Moscow, Russia, [email protected] 5. IOTA, Murrumbateman, NSW, Australia, [email protected] 6. IOTA, Forest Glen, MD, USA, [email protected] 7. Carl Sagan Center at the SETI Institute, 189 Bernardo Av, Mountain View CA 94043, USA, [email protected] Corresponding author Franck Marchis Carl Sagan Center at the SETI Institute 189 Bernardo Av Mountain View CA 94043 USA [email protected] 1 Keywords: Asteroids, Binary Asteroids, Trojan Asteroids, Occultation Abstract: On 2012 January 19, observers in the northeastern United States of America observed an occultation of 8.0-mag HIP 41337 star by the Jupiter-Trojan (911) Agamemnon, including one video recorded with a 36cm telescope that shows a deep brief secondary occultation that is likely due to a satellite, of about 5 km (most likely 3 to 10 km) across, at 278 km ±5 km (0.0931″) from the asteroid’s center as projected in the plane of the sky. A satellite this small and this close to the asteroid could not be resolved in the available VLT adaptive optics observations of Agamemnon recorded in 2003. -

Thermal-IR Spectral Analysis of Jupiter's Trojan Asteroids
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 1238.pdf THERMAL-IR SPECTRAL ANALYSIS OF JUPITER’S TROJAN ASTEROIDS: DETECTING SILICATES. A. C. Martin1, J. P. Emery1, S. S. Lindsay2, 1The University of Tennessee Earth and Planetary Science Department, 1621 Cumberland Avenue, 602 Strong Hall, Knoxville TN, 37996, 2The University of Tennessee, De- partment of Physics, 1408 Circle Drive, Knoxville TN, 37996.. Introduction: Jupiter’s Trojan asteroids (hereafter (e.g., [11],[8]). Had Trojans and JFCs formed in the Trojans) populate Jupiter’s L4 and L5 Lagrange points. same region, Trojans should have fine-grained silicates The L4 and L5 points are dynamically stable over the in primarily amorphous phases. lifetime of the Solar System, and, therefore, Trojans Analysis of TIR spectra by [12] shows that the sur- could have resided in the L4 and L5 regions for nearly faces of three Trojans (624 Hektor, 1172 Aneas, and 911 4.5 Gyr [1]. However, it is still uncertain where the Tro- Agamemnon) have emissivity features similar to fine- jans formed and when they were captured. Asteroid or- grained silicates in comet comae. The TIR wavelength igins provide an effective means of constraining the region is beneficial for silicate mineralogy detection be- events that dynamically shaped the solar system. Tro- cause it contains fundamental Si-O molecular vibrations jans may help in determining the extent of radial mixing (stretching at 9 –12 µm and bending at 14 – 25 µm; that occurred during giant planet migration. [13]). Comets produce optically thin comae that result Trojans are thought to have formed in one of two in strong 10-µm emission features when comprised of locations: (1) in their current position (~5.2 AU), or (2) fine-grained (≤10 to 20 µm) dispersed silicates. -

On the Accuracy of Restricted Three-Body Models for the Trojan Motion
DISCRETE AND CONTINUOUS Website: http://AIMsciences.org DYNAMICAL SYSTEMS Volume 11, Number 4, December 2004 pp. 843{854 ON THE ACCURACY OF RESTRICTED THREE-BODY MODELS FOR THE TROJAN MOTION Frederic Gabern1, Angel` Jorba1 and Philippe Robutel2 Departament de Matem`aticaAplicada i An`alisi Universitat de Barcelona Gran Via 585, 08007 Barcelona, Spain1 Astronomie et Syst`emesDynamiques IMCCE-Observatoire de Paris 77 Av. Denfert-Rochereau, 75014 Paris, France2 Abstract. In this note we compare the frequencies of the motion of the Trojan asteroids in the Restricted Three-Body Problem (RTBP), the Elliptic Restricted Three-Body Problem (ERTBP) and the Outer Solar System (OSS) model. The RTBP and ERTBP are well-known academic models for the motion of these asteroids, and the OSS is the standard model used for realistic simulations. Our results are based on a systematic frequency analysis of the motion of these asteroids. The main conclusion is that both the RTBP and ERTBP are not very accurate models for the long-term dynamics, although the level of accuracy strongly depends on the selected asteroid. 1. Introduction. The Restricted Three-Body Problem models the motion of a particle under the gravitational attraction of two point masses following a (Keple- rian) solution of the two-body problem (a general reference is [17]). The goal of this note is to discuss the degree of accuracy of such a model to study the real motion of an asteroid moving near the Lagrangian points of the Sun-Jupiter system. To this end, we have considered two restricted three-body problems, namely: i) the Circular RTBP, in which Sun and Jupiter describe a circular orbit around their centre of mass, and ii) the Elliptic RTBP, in which Sun and Jupiter move on an elliptic orbit. -

Structure and Composition of the Surfaces of Trojan Asteroids from Reflection and Emission Spectroscopy
Lunar and Planetary Science XXXVII (2006) 2075.pdf STRUCTURE AND COMPOSITION OF THE SURFACES OF TROJAN ASTEROIDS FROM REFLECTION AND EMISSION SPECTROSCOPY. Joshua. P. Emery,1 Dale. P. Cruikshank,2 and Jeffrey Van Cleve3 1NASA Ames / SETI Institute ([email protected]), 2NASA Ames Research Center ([email protected]), 3 Ball Aerospace ([email protected]). Introduction: The orbits of Trojan asteroids (~5.2 AU – beyond the Main Belt) place them in the transi- 1.0 tion region between the rocky inner and icy outer Solar 0.9 1172 Aneas System. Most Trojans were traditionally thought to 0.8 have originated in this region [3], although other loca- 1.0 tions of origin are possible [e.g., 4,5,6]. Possible con- 0.9 nections between Trojans and other groups of objects 911 Agamemnon 0.8 (Jupiter family comets, irregular satellites, Centaurs, Emissivity KBOs) are also important, but only poorly understood 1.0 [4,6,7,9]. The compositions of Trojans thereby hold 0.9 624 Hektor important clues concerning conditions in this critical 0.8 transition region, and the solar nebula as a whole. We discuss emission and reflection spectra of three Trojans 10 15 20 25 30 35 Wavelength (µm) (624 Hektor, 911 Agamemnon, and 1172 Aneas) and implications for surface structure and composition. Figure 2. Mid-IR emissivity spectra of Trojans. Vis-NIR Reflectance Spectroscopy: Reflectance studies of Trojans in the visible and NIR (0.8 – 4.0 Analysis: The Trojans have a similar spectral shape µm) reveal dark surfaces with mild to very red spectral to some carbonaceous meteorites and fine-grained sili- slopes, but no distinct absorption features (Fig. -

Astrocladistics of the Jovian Trojan Swarms
MNRAS 000,1–26 (2020) Preprint 23 March 2021 Compiled using MNRAS LATEX style file v3.0 Astrocladistics of the Jovian Trojan Swarms Timothy R. Holt,1,2¢ Jonathan Horner,1 David Nesvorný,2 Rachel King,1 Marcel Popescu,3 Brad D. Carter,1 and Christopher C. E. Tylor,1 1Centre for Astrophysics, University of Southern Queensland, Toowoomba, QLD, Australia 2Department of Space Studies, Southwest Research Institute, Boulder, CO. USA. 3Astronomical Institute of the Romanian Academy, Bucharest, Romania. Accepted XXX. Received YYY; in original form ZZZ ABSTRACT The Jovian Trojans are two swarms of small objects that share Jupiter’s orbit, clustered around the leading and trailing Lagrange points, L4 and L5. In this work, we investigate the Jovian Trojan population using the technique of astrocladistics, an adaptation of the ‘tree of life’ approach used in biology. We combine colour data from WISE, SDSS, Gaia DR2 and MOVIS surveys with knowledge of the physical and orbital characteristics of the Trojans, to generate a classification tree composed of clans with distinctive characteristics. We identify 48 clans, indicating groups of objects that possibly share a common origin. Amongst these are several that contain members of the known collisional families, though our work identifies subtleties in that classification that bear future investigation. Our clans are often broken into subclans, and most can be grouped into 10 superclans, reflecting the hierarchical nature of the population. Outcomes from this project include the identification of several high priority objects for additional observations and as well as providing context for the objects to be visited by the forthcoming Lucy mission. -

Trajectory Design of the Lucy Mission to Explore the Diversity of the Jupiter Trojans
70th International Astronautical Congress, Washington, DC. This material is declared a work of the U.S. Government and is not subject to copyright protection in the United States. IAC–2019–C1.2.11 Trajectory Design of the Lucy Mission to Explore the Diversity of the Jupiter Trojans Jacob A. Englander Aerospace Engineer, Navigation and Mission Design Branch, NASA Goddard Space Flight Center Kevin Berry Lucy Flight Dynamics Lead, Navigation and Mission Design Branch, NASA Goddard Space Flight Center Brian Sutter Totally Awesome Trajectory Genius, Lockheed Martin Space Systems, Littleton, CO Dale Stanbridge Lucy Navigation Team Chief, KinetX Aerospace, Simi Valley, CA Donald H. Ellison Aerospace Engineer, Navigation and Mission Design Branch, NASA Goddard Space Flight Center Ken Williams Flight Director, Space Navigation and Flight Dynamics Practice, KinetX Aerospace, Simi Valley, California James McAdams Aerospace Engineer, Space Navigation and Flight Dynamics Practice, KinetX Aerospace, Simi Valley, California Jeremy M. Knittel Aerospace Engineer, Space Navigation and Flight Dynamics Practice, KinetX Aerospace, Simi Valley, California Chelsea Welch Fantastically Awesome Deputy Trajectory Genius, Lockheed Martin Space Systems, Littleton, CO Hal Levison Principle Investigator, Lucy mission, Southwest Research Institute, Boulder, CO Lucy, NASA’s next Discovery-class mission, will explore the diversity of the Jupiter Trojan asteroids. The Jupiter Trojans are thought to be remnants of the early solar system that were scattered inward when the gas giants migrated to their current positions as described in the Nice model. There are two stable subpopulations, or “swarms,” captured at the Sun-Jupiter L4 and L5 regions. These objects are the most accessible samples of what the outer solar system may have originally looked like. -

(624) Hektor F
Asteroids, Comets, Meteors (2012) 6416.pdf PHYSICAL CHARACTERISTICS OF THE BINARY TROJAN ASTEROID (624) HEKTOR F. Marchis1,2, F. Vachier2, J. Durech3, J. Berthier2, M.H. Wong4, P. Kalas4, G. Duchene4,5,M. van Dam6, 1SETI Insti- tute ([email protected]) Carl Sagan Center, Mountain View CA USA, 2.IMCCE, Observatoire de Paris, France 3Astronomical Institute, Charles University in Prague, CzR 4Department of Asronomy, University of California, Berkeley USA, 5Observatoire de Grenoble, France 6. Flat Wavefronts, Christchurch, NZ Introduction: In July 2006, [1] announced the primary [10], we will derive the mass, hence the bulk discovery of a 15-km diameter satellite around the L4 density of Hektor. Jupiter Trojan asteroid (624) Hektor from direct obser- Preliminary Results: Genoid, genetic-based algo- vations using the W.M. Keck telescope. Over the past rithm [10], finds several prelimary solutions with ac- 6 years, our group has collected additional observa- ceptable fitting parameter 25<fp<50 milli-arcsec using tions to pinpoint the orbital parameters of Hektor’s a purely Keplerian orbit. All solutions have a period satellite, derive the mass of the system and thus infer P~2.98 days, a semi-major axis a between 1,100 and the average density of this large (Deq~225 km) Trojan 1,400 km and a significant eccentricity (e=0.13-0.20) asteroid. The density of the L5 double Trojan (617) which suggests an excited orbit (e.g. [4]). Using 3 Patroclus, estimated to be between 0.7-1.0 g/cm by [2] Deq~225 km, we derive a large density between 1.8 and (from IRAS) or 0.8-1.4 g/cm3 by [3], is the only den- 3.5 g/cm3. -
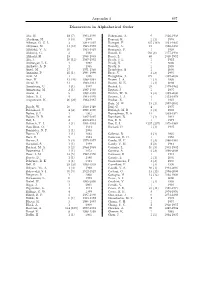
Appendix 1 897 Discoverers in Alphabetical Order
Appendix 1 897 Discoverers in Alphabetical Order Abe, H. 22 (7) 1993-1999 Bohrmann, A. 9 1936-1938 Abraham, M. 3 (3) 1999 Bonomi, R. 1 (1) 1995 Aikman, G. C. L. 3 1994-1997 B¨orngen, F. 437 (161) 1961-1995 Akiyama, M. 14 (10) 1989-1999 Borrelly, A. 19 1866-1894 Albitskij, V. A. 10 1923-1925 Bourgeois, P. 1 1929 Aldering, G. 3 1982 Bowell, E. 563 (6) 1977-1994 Alikoski, H. 13 1938-1953 Boyer, L. 40 1930-1952 Alu, J. 20 (11) 1987-1993 Brady, J. L. 1 1952 Amburgey, L. L. 1 1997 Brady, N. 1 2000 Andrews, A. D. 1 1965 Brady, S. 1 1999 Antal, M. 17 1971-1988 Brandeker, A. 1 2000 Antonini, P. 25 (1) 1996-1999 Brcic, V. 2 (2) 1995 Aoki, M. 1 1996 Broughton, J. 179 1997-2002 Arai, M. 43 (43) 1988-1991 Brown, J. A. 1 (1) 1990 Arend, S. 51 1929-1961 Brown, M. E. 1 (1) 2002 Armstrong, C. 1 (1) 1997 Broˇzek, L. 23 1979-1982 Armstrong, M. 2 (1) 1997-1998 Bruton, J. 1 1997 Asami, A. 5 1997-1999 Bruton, W. D. 2 (2) 1999-2000 Asher, D. J. 9 1994-1995 Bruwer, J. A. 4 1953-1970 Augustesen, K. 26 (26) 1982-1987 Buchar, E. 1 1925 Buie, M. W. 13 (1) 1997-2001 Baade, W. 10 1920-1949 Buil, C. 4 1997 Babiakov´a, U. 4 (4) 1998-2000 Burleigh, M. R. 1 (1) 1998 Bailey, S. I. 1 1902 Burnasheva, B. A. 13 1969-1971 Balam, D. -

Society Amateur Astronomy News and Views in Southwestern Virginia
Roanoke Valley Astronomical Society Amateur Astronomy News and Views In Southwestern Virginia Volume 31—Number 2 February 2014 RVAS January Meeting Notes Coming to you live . by Rick Rader, RVAS Secretary From Seattle, Washington, it's--wait, wait, don't tell me! Sorry to steal a line from the Sunday morning NPR pro- gram, but our January Club gathering was yet another example of why you may wish to brave the elements to meet with your fellow all-things-astronomical enthusi- asts. In a first for a monthly meeting, RVAS President Frank Baratta and Mark Hodges (all things mystical and elec- trical Science Museum master) orchestrated an intri- guing hour with Tom Field, live via webcast from his lo- cation in Seattle. A contributing editor at Sky & Tele- scope magazine and author of RSpec software, Tom re- Frank Baratta Tom Field galed the twenty eight hardy souls present with an ad- RVAS President connects with for the evening’s live webcast talk. venture delving into the surprisingly easy world of hard Photo by John Goss science: spectroscopic investigation of light emitting objects of all types in our universe. had been born. The bombardment of energy reaching Earth can be ana- Fast forward. Spectroscopy has taught us that every lyzed to reveal the constituent elements of a celestial element of the Periodic Table when heated emits or ab- object. The process is not brand new, having been first sorbs light of specific wavelengths, which appear as defined by Newton with his prism experiments. Joseph lines in their otherwise continuous, multi-colored spec- von Fraunhofer, a wonderfully skilled glass maker, ob- tra. -

Binary Structures Among Large Asteroids
A&A 383, L19–L22 (2002) Astronomy DOI: 10.1051/0004-6361:20020015 & c ESO 2002 Astrophysics Binary structures among large asteroids M. Kaasalainen, J. Torppa, and J. Piironen Observatory, University of Helsinki, PO Box 14, 00014 Helsinki, Finland Received 19 November 2001 / Accepted 2 January 2002 Abstract. The imaging of well-observed large asteroids by lightcurve inversion has revealed objects, such as 44 Nysa and 41 Daphne, that are distinctly asymmetric and globally very different from equilibrium-like figures, indicating contact-binary structures. The shape of the Trojan asteroid 624 Hektor is very probably globally bifurcated. Together with the known large binary asteroids 216 Kleopatra, 90 Antiope, and 617 Patroclus, these findings indicate that a nonvanishing portion of large asteroids have binary structures. Key words. solar system: minor planets, asteroids – techniques: photometric – methods: numerical 1. Introduction with the morphology and evolutionary histories ex- pected of asteroidal bodies composed of predominantly Lightcurve inversion is an efficient deconvolution tech- homogeneous material. Some large asteroids, however, nique whose resolving capacity lies between space tele- stand out from the majority. They display distinctly scope and radar, and whose range extends from near- asymmetric shapes; even more remarkable, these asym- Earth to main-belt asteroids (Kaasalainen & Torppa 2001; metries are of quite regular kind. Asteroids 44 Nysa Kaasalainen et al. 2001; Kaasalainen 2001). The rotational and 41 Daphne, with IRAS size estimates, respectively, state and the characteristic large-scale shape features of some 70 km and 180 km (Small Bodies Data Archives: an object (such as those of 6489 Golevka or 433 Eros) can http://pdssbn.astro.umd.edu/sbnhtml/index.html# well be inferred from good-quality data by lightcurve in- asteroids) are the foremost examples of such objects. -

Trajectory Optimization for a Misson to the Trojan Asteroids
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 8-2014 Trajectory Optimization for a Misson to the Trojan Asteroids Shivaji Senapati Gadsing Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Astrodynamics Commons, Navigation, Guidance, Control and Dynamics Commons, and the Space Vehicles Commons Recommended Citation Gadsing, Shivaji Senapati, "Trajectory Optimization for a Misson to the Trojan Asteroids" (2014). Master's Theses. 523. https://scholarworks.wmich.edu/masters_theses/523 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. TRAJECTORY OPTIMIZATION FOR A MISSON TO THE TROJAN ASTEROIDS by Shivaji Senapati Gadsing A thesis submitted to the Graduate College in partial fulfillment of the requirements for the Degree of Master of Science Mechanical and Aerospace Engineering Western Michigan University August 2014 Thesis Committee: Jennifer Hudson, Ph.D., Chair James Kamman, Ph.D. Kapseong Ro, Ph.D. Christopher Cho, Ph.D. TRAJECTORY OPTIMIZATION FOR A MISSON TO THE TROJAN ASTEROIDS Shivaji Senapati Gadsing, M.S. Western Michigan University, 2014 The problem of finding a minimum-fuel trajectory for a mission to the Jovian Trojan asteroids is considered. The problem is formulated as a modified traveling salesman problem. Two different types of algorithms such as an exhaustive search algorithm and a serial rendezvous search algorithm are developed. The General Mission Analysis Tool (GMAT) is employed for finding optimum trajectories with minimal fuel consumption. -

Solar System Science with the Wide-Field Infrared Survey Telescope (WFIRST)
Solar system science with the Wide-Field InfraRed Survey Telescope (WFIRST) Holler, B. J., Milam, S. N., Bauer, J. M., Alcock, C., Bannister, M. T., Bjoraker, G. L., Bodewits, D., Bosh, A. S., Buie, M. W., Farnham, T. L., Haghighipour, N., Hardersen, P. S., Harris, A. W., Hirata, C. M., Hsieh, H. H., Kelley, M. S. P., Knight, M. M., Kramer, E. A., Longobardo, A., ... West, R. A. (2017). Solar system science with the Wide-Field InfraRed Survey Telescope (WFIRST). Proceedings of SPIE. Published in: Proceedings of SPIE Document Version: Peer reviewed version Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright © 2018 SPIE. This work is made available online in accordance with the publisher’s policies. Please refer to any applicable terms of use of the publisher. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:11. Oct. 2021 Solar system science with the Wide-Field InfraRed Survey Telescope (WFIRST) B.J.