Beyond Type 1 and Type 2 Diabetes; Why Correct Diabetes Classification Is Important Gabriel I. Uwaifo, MD Dept of Endocrinology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Studies of Streptozotocin-Induced Insulitis and Diabetes
Proc. NatI. Acad. Sci. USA Vol. 74, No. 6, pp. 2485-2489, June 1977 "I ", .4-4" I- Cell Biology ,- -i,' Studies of streptozotocin-induced insulitis and diabetes (pancreatic beta cells/islets of Langerhans/alloxan diabetes/type C virus induction/cell-mediated reaction) ALDO A. ROSSINI*, ARTHUR A. LIKEt, WILLIAM L. CHICK*, MICHAEL C. APPELt, AND GEORGE F. CAHILL, JR. * * Joslin Research Laboratory, One Joslin Place, Boston, Massachusetts 02215 and Harvard Medical School and Peter Bent Brigham Hospital, Boston, Massachusetts 02215; and t Department of Pathology, University of Massachusetts Medical School, Worcester, Massachusetts 01605 Communicated by Eugene Braunwald, April 4, 1977 ABSTRACT Multiple small injections of streptozotocin was a subsequent decrease in inflammation within the re- produce a delayed, progressive increase in plasma-glucose in maining islets which were small and composed almost exclu- mice within 5-6 days after the injections, in association with sively of non-beta cells. Blood glucose values remained in the pronounced insulitis and induction of type C viruses within beta cells. Multiple subdiabetogenic doses of streptozotocin in rats diabetic range and correlated well with the pathologic changes and multiple injections of another beta cell toxin, alloxan, in within the islets. Noteworthy also was the presence of numerous mice did not induce insulitis although hyperglycemia followed type C viruses within the surviving beta cells of animals studied the injection of larger quantities of both agents. In mice, the within 6 days of the last SZ injection. The delayed development prior injection of 3-O-methyl-D-glucose (3-OMG) or nicotin- of the insulitis and the nature of the inflammatory infiltrate amide attenuated the diabetic syndrome produced by strepto- zotocin; however, 3-OMG was more protective. -

Multiple Low-Dose Streptozotocin-Induced Diabetes in the Mouse
Multiple low-dose streptozotocin-induced diabetes in the mouse. Evidence for stimulation of a cytotoxic cellular immune response against an insulin-producing beta cell line. R C McEvoy, … , S Sandler, C Hellerström J Clin Invest. 1984;74(3):715-722. https://doi.org/10.1172/JCI111487. Research Article Mice were examined for the presence of splenocytes specifically cytotoxic for a rat insulinoma cell line (RIN) during the induction of diabetes by streptozotocin (SZ) in multiple low doses (Multi-Strep). Cytotoxicity was quantitated by the release of 51Cr from damaged cells. A low but statistically significant level of cytolysis (5%) by splenocytes was first detectable on day 8 after the first dose of SZ. The cytotoxicity reached a maximum of approximately 9% on day 10 and slowly decreased thereafter, becoming undetectable 42 d after SZ was first given. The time course of the in vitro cytotoxic response correlated with the degree of insulitis demonstrable in the pancreata of the Multi-Strep mice. The degree of cytotoxicity after Multi-Strep was related to the number of effector splenocytes to which the target RIN cells were exposed and was comparable to that detectable after immunization by intraperitoneal injection of RIN cells in normal mice. The cytotoxicity was specific for insulin-producing cells; syngeneic, allogeneic, and xenogeneic lymphocytes and lymphoblasts, 3T3 cells, and a human keratinocyte cell line were not specifically lysed by the splenocytes of the Multi- Strep mice. This phenomenon was limited to the Multi-Strep mice. Splenocytes from mice made diabetic by a single, high dose of SZ exhibited a very low level of cytotoxicity against the RIN cells. -

Jeffrey Kleinberger Ph.D. Candidate Molecular Medicine Program, Genome Biology Track Graduate Program in Life Sciences, University of Maryland, Baltimore
Discovery and Analysis of Patients with Monogenic Diabetes in Multiple Cohorts to Guide Future Diagnosis Item Type dissertation Authors Kleinberger, Jeffrey Publication Date 2017 Abstract Monogenic diabetes is hyperglycemia caused by a variant in a single gene, and it accounts for approximately 1-2% of all diabetes cases. A genetic diagnosis of monogenic diabetes is important because the most common gene etiologies can be effectively ... Keywords MODY; monogenic diabetes; screening; Clinical Trial; Diabetes Mellitus, Type 2; Zebrafish Download date 05/10/2021 09:00:42 Link to Item http://hdl.handle.net/10713/7072 Jeffrey Kleinberger Ph.D. Candidate Molecular Medicine Program, Genome Biology Track Graduate Program in Life Sciences, University of Maryland, Baltimore Contact Information Medical School Teaching Facility 357 685 West Baltimore St. Baltimore, MD 21201 [email protected] Education Juniata College Huntingdon, PA August 2004 – May 2008 Cum Laude distinction (GPA: 3.701) Biochemistry major Coursework included: Organic Chemistry, Bioinorganic Chemistry, Analytical Chemistry, Physical Chemistry, Chemistry Research, Biology, Cell Biology, Genetics, Biostatistics, Molecular Techniques, Physiology, Microbiology, Biochemistry and Molecular Biology (I-III), Calculus (I&II), Physics (I&II), Scientific Glassblowing, College Writing Seminar, Art of Public Speaking, Intro to Psychology, Learning & Conditioning, Mod. Knowledge & The Self, United States to 1877, The American Revolution, Survey of Western Art, Modern Architecture, -

一般演題(口演)/Oral Sessions
一般演題(口演)!Oral Sessions ご ! 案 第 60 回大会賞候補セッション(口演) Oral Presentation Award Session 内 日 時:10 月 16 日(金)11:25~12:20 第 2 会場(南館 4 階 錦) 座 長:稲澤 譲治(東京医科歯科大学難治疾患研究所ゲノム応用医学研究部門(分子細胞遺伝)) 田中 敏博(東京医科歯科大学疾患バイオリソースセンター) Date:Oct. 16 (Fri.) 11:25~12:20 Room 2(Nishiki, South Tower 4F) Chairs:Johji Inazawa(Tokyo Medical and Dental University) Toshihiro Tanaka(Tokyo Medical and Dental University) プ ロ BO-1 ゲノムワイド関連解析(GWAS)データを活用した in silico 解析による新規抗パーキンソン病薬 グ の探索 ラ In silico drug discovery for Parkinson’s disease by using genome!wide association study ム (GWAS)data ○上中 健 1(Takeshi Uenaka)、佐竹 渉 1(Wataru Satake)、謝 珮琴 1(Pei"Chieng Cha)、 岡田 随象 2(Yukinori Okada)、戸田 達史 1(Tatsushi Toda) 1 神戸大学大学院医学研究科神経内科!分子脳科学 指 (Division of Neurology, Kobe University Graduate School of Medicine, Kobe, Japan) 定 2 東京医科歯科大学大学院医歯学総合研究科疾患多様性遺伝学分野 演 (Department of Human Genetics and Disease Diversity, Graduate School of Medical and Dental Sciences, 題 Tokyo Medical and Dental University, Tokyo, Japan) 抄 録 BO-2 MIRAGE 症候群:機能亢進型 SAMD9 変異を原因とする新規症候群の発見 MIRAGE syndrome:New genetic syndrome caused by activating SAMD9 mutations ○鳴海 覚志 1(Satoshi Narumi)、天野 直子 1(Naoko Amano)、石井 智弘 1(Tomohiro Ishii)、 一 勝又 規行 2(Noriyuki Katsumata)、福澤 龍二 3(Ryuji Fukuzawa)、芝田 晋介 4(Shinsuke Shibata)、 般 4 5 6 演 岡野 栄之 (Hideyuki Okano)、清水 厚志 (Atsushi Shimizu)、三宅 紀子 (Noriko Miyake)、 題 松本 直通 6(Naomichi Matsumoto)、長谷川 奉延 1(Tomonobu Hasegawa) ( 1 慶應義塾大学医学部小児科 口 (Department of Pediatrics, Keio University School of Medicine, Tokyo, Japan) 演 ) 2 国立成育医療研究センター研究所分子内分泌研究部 抄 (Department of Molecular Endocrinology, National Research -

Residual Β Cell Function and Monogenic Variants in Long- Duration Type 1 Diabetes Patients
Residual β cell function and monogenic variants in long- duration type 1 diabetes patients Marc Gregory Yu, … , Marcus G. Pezzolesi, George Liang King J Clin Invest. 2019. https://doi.org/10.1172/JCI127397. Clinical Medicine Endocrinology Metabolism In the Joslin Medalist Study (Medalists), we determined whether significant associations exist between β cell function and pathology and clinical characteristics. Individuals with type 1 diabetes (T1D) for 50 or more years underwent evaluation including HLA analysis, basal and longitudinal autoantibody (AAb) status, and β cell function by a mixed-meal tolerance test (MMTT) and a hyperglycemia/arginine clamp procedure. Postmortem analysis of pancreases from 68 Medalists was performed. Monogenic diabetes genes were screened for the entire cohort. Of the 1019 Medalists, 32.4% retained detectable C-peptide levels (>0.05 ng/mL, median: 0.21 ng/mL). In those who underwent a MMTT (n = 516), 5.8% responded with a doubling of baseline C-peptide levels. Longitudinally n( = 181, median: 4 years), C-peptide levels increased in 12.2% (n = 22) and decreased in 37% (n = 67) of the Medalists. Among those with repeated MMTTs, 5.4% (3 of 56) and 16.1% (9 of 56) had waxing and waning responses, respectively. Thirty Medalists with baseline C-peptide levels of 0.1 ng/mL or higher underwent the clamp procedure, with HLA–/AAb– and HLA+/AAb– Medalists being most responsive. Postmortem examination of pancreases from 68 Medalists showed that all had scattered insulin-positive […] Find the latest version: https://jci.me/127397/pdf The Journal of Clinical Investigation CLINICAL MEDICINE Residual β cell function and monogenic variants in long-duration type 1 diabetes patients Marc Gregory Yu,1,2 Hillary A. -

Impaired Hormonal Responses to Hypoglycemia in Spontaneously Diabetic and Recurrently Hypoglycemic Rats
Impaired hormonal responses to hypoglycemia in spontaneously diabetic and recurrently hypoglycemic rats. Reversibility and stimulus specificity of the deficits. A M Powell, … , R S Sherwin, G I Shulman J Clin Invest. 1993;92(6):2667-2674. https://doi.org/10.1172/JCI116883. Research Article To evaluate the roles of iatrogenic hypoglycemia and diabetes per se in the pathogenesis of defective hormonal counterregulation against hypoglycemia in insulin-dependent diabetes mellitus (IDDM), nondiabetic, and spontaneously diabetic BB/Wor rats were studied using a euglycemic/hypoglycemic clamp. In nondiabetic rats, recurrent (4 wk) insulin- induced hypoglycemia (mean daily glucose, MDG, 59 mg/dl) dramatically reduced glucagon and epinephrine responses by 84 and 94%, respectively, to a standardized glucose fall from 110 to 50 mg/dl. These deficits persisted for > 4 d after restoring normoglycemia, and were specific for hypoglycemia, with normal glucagon and epinephrine responses to arginine and hypovolemia, respectively. After 4 wk of normoglycemia, hormonal counterregulation increased, with the epinephrine, but not the glucagon response reaching control values. In diabetic BB rats (MDG 245 mg/dl with intermittent hypoglycemia), glucagon and epinephrine counterregulation were reduced by 86 and 90%, respectively. Chronic iatrogenic hypoglycemia (MDG 52 mg/dl) further suppressed counterregulation. Prospective elimination of hypoglycemia (MDG 432 mg/dl) improved, but did not normalize hormonal counterregulation. In diabetic rats, the glucagon defect appeared to be specific for hypoglycemia, whereas deficient epinephrine secretion also occurred during hypovolemia. We concluded that both recurrent hypoglycemia and the diabetic state independently lead to defective hormonal counterregulation. These data suggest that in IDDM iatrogenic hypoglycemia magnifies preexisting counterregulatory defects, thereby increasing the risk of severe hypoglycemia. -

The Myotonic Dystrophies: Diagnosis and Management Chris Turner,1 David Hilton-Jones2
Review J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2008.158261 on 22 February 2010. Downloaded from The myotonic dystrophies: diagnosis and management Chris Turner,1 David Hilton-Jones2 1Department of Neurology, ABSTRACT asymptomatic relatives as well as prenatal and National Hospital for Neurology There are currently two clinically and molecularly defined preimplantation diagnosis can also be performed.7 and Neurosurgery, London, UK 2Department of Clinical forms of myotonic dystrophy: (1) myotonic dystrophy Neurology, The Radcliffe type 1 (DM1), also known as ‘Steinert’s disease’; and Anticipation Infirmary, Oxford, UK (2) myotonic dystrophy type 2 (DM2), also known as DMPK alleles greater than 37 CTG repeats in length proximal myotonic myopathy. DM1 and DM2 are are unstable and may expand in length during meiosis Correspondence to progressive multisystem genetic disorders with several and mitosis. Children of a parent with DM1 may Dr C Turner, Department of Neurology, National Hospital for clinical and genetic features in common. DM1 is the most inherit repeat lengths considerably longer than those Neurology and Neurosurgery, common form of adult onset muscular dystrophy whereas present in the transmitting parent. This phenomenon Queen Square, London WC1N DM2 tends to have a milder phenotype with later onset of causes ‘anticipation’, which is the occurrence of 3BG, UK; symptoms and is rarer than DM1. This review will focus increasing disease severity and decreasing age of onset [email protected] on the clinical features, diagnosis and management of in successive generations. The presence of a larger Received 1 December 2008 DM1 and DM2 and will briefly discuss the recent repeat leads to earlier onset and more severe disease Accepted 18 December 2008 advances in the understanding of the molecular and causes the more severe phenotype of ‘congenital’ pathogenesis of these diseases with particular reference DM1 (figure 2).8 9 A child with congenital DM 1 to new treatments using gene therapy. -

Gut Microbiota Differs in Composition and Functionality Between Children
Diabetes Care Volume 41, November 2018 2385 Gut Microbiota Differs in Isabel Leiva-Gea,1 Lidia Sanchez-Alcoholado,´ 2 Composition and Functionality Beatriz Mart´ın-Tejedor,1 Daniel Castellano-Castillo,2,3 Between Children With Type 1 Isabel Moreno-Indias,2,3 Antonio Urda-Cardona,1 Diabetes and MODY2 and Healthy Francisco J. Tinahones,2,3 Jose´ Carlos Fernandez-Garc´ ´ıa,2,3 and Control Subjects: A Case-Control Mar´ıa Isabel Queipo-Ortuno~ 2,3 Study Diabetes Care 2018;41:2385–2395 | https://doi.org/10.2337/dc18-0253 OBJECTIVE Type 1 diabetes is associated with compositional differences in gut microbiota. To date, no microbiome studies have been performed in maturity-onset diabetes of the young 2 (MODY2), a monogenic cause of diabetes. Gut microbiota of type 1 diabetes, MODY2, and healthy control subjects was compared. PATHOPHYSIOLOGY/COMPLICATIONS RESEARCH DESIGN AND METHODS This was a case-control study in 15 children with type 1 diabetes, 15 children with MODY2, and 13 healthy children. Metabolic control and potential factors mod- ifying gut microbiota were controlled. Microbiome composition was determined by 16S rRNA pyrosequencing. 1Pediatric Endocrinology, Hospital Materno- Infantil, Malaga,´ Spain RESULTS 2Clinical Management Unit of Endocrinology and Compared with healthy control subjects, type 1 diabetes was associated with a Nutrition, Laboratory of the Biomedical Research significantly lower microbiota diversity, a significantly higher relative abundance of Institute of Malaga,´ Virgen de la Victoria Uni- Bacteroides Ruminococcus Veillonella Blautia Streptococcus versityHospital,Universidad de Malaga,M´ alaga,´ , , , , and genera, and a Spain lower relative abundance of Bifidobacterium, Roseburia, Faecalibacterium, and 3Centro de Investigacion´ BiomedicaenRed(CIBER)´ Lachnospira. -

X-Linked Myotubular Myopathy and Chylothorax
ARTICLE IN PRESS Neuromuscular Disorders xxx (2007) xxx–xxx www.elsevier.com/locate/nmd Case report X-linked myotubular myopathy and chylothorax Koenraad Smets * Department of Neonatology, Ghent University Hospital, De Pintelaan 185, B-9000 Ghent, Belgium Received 10 August 2007; received in revised form 4 October 2007; accepted 24 October 2007 Abstract X-linked myotubular myopathy usually presents at birth with hypotonia and respiratory distress. Phenotypic presentation, however, can be extreme variable. We report on a newborn baby, who presented with the severe form of the disease. In the second week of life, he developed a clinically relevant chylothorax, needing drainage and treatment with octreotide acetate. Pleural effusions are frequently described in patients with congenital myotonic dystrophy. To our knowledge, the association of chylothorax and X-linked myotubular myopathy has not been described to date. As chylothorax could not be attributed to any evident condition in this child, perhaps it may be added to the clinical spectrum of X-linked myotubular myopathy. Ó 2007 Elsevier B.V. All rights reserved. Keywords: X-linked myotubular myopathy; Chylothorax 1. Introduction drainage (Fig. 1). Laboratory examination was compatible with chylothorax (5230 white blood cells/ll, 98% lympho- Congenital myopathies often present with hypotonia cytes; chylomicrons were present; triglycerides 746 mg/dl). and respiratory distress from birth, although their expres- There were no central venous catheters in place who could sion may be delayed. In most cases muscle biopsy is war- have caused thrombosis, impairing lymphatic flow, neither ranted for definitive diagnosis. In some instances could any other risk factor for chylothorax be identified. -

Functional Imaging of Insulitis in Type 1 Diabetes
FUNCTIONAL IMAGING OF INSULITIS IN TYPE 1 DIABETES Teemu Kalliokoski TURUN YLIOPISTON JULKAISUJA – ANNALES UNIVERSITATIS TURKUENSIS Sarja - ser. D osa - tom. 1135 | Medica - Odontologica | Turku 2014 University of Turku Faculty of Medicine Department of Clinical Medicine Pediatrics and Turku PET Centre Doctoral Programme of Clinical Investigation Supervised by Professor emeritus Olli Simell (MD, PhD) Adjunct professor Merja Haaparanta-Solin (MSc, PhD) University of Turku, Faculty of Medicine University of Turku, Faculty of Medicine Department of Pediatrics Turku PET Centre Reviewed by Professor Timo Otonkoski (MD, PhD) Docent Olof Eriksson (MSc, PhD) University of Helsinki Uppsala University Children’s Hospital Department of Medicinal Chemistry Opponent Professor Alberto Signore (MD, PhD) Sapienza University Nuclear Medicine Unit Rome, Italy The originality of this thesis has been checked in accordance with the University of Turku quality assurance system using the Turnitin OriginalityCheck service. ISBN 978-951-29-5859-7 (PRINT) ISBN 978-951-29-5860-3 (PDF) ISSN 0355-9483 Painosalama Oy - Turku, Finland 2014 Adjunct professor Merja Haaparanta-Solin (MSc, PhD) University of Turku, Faculty of Medicine Turku PET Centre To Mervi, and our children Elias and Iiris 4 Abstract ABSTRACT Teemu Kalliokoski FUNCTIONAL IMAGING OF INSULITIS IN TYPE 1 DIABETES Department of Pediatrics and Turku PET Centre, Faculty of Medicine, University of Turku Annales Universitatis Turkuensis, 2014, Turku, Finland Type 1 diabetes (T1D) is an immune-mediated disease characterized by autoimmune inflammation (insulitis), leading to destruction of insulin-producing pancreatic β-cells and consequent dependence on exogenous insulin. Current evidence suggests that T1D arises in genetically susceptible children who are exposed to poorly characterized environmental triggers. -

Insulitis and Diabetes: a Perspective on Islet Inflammation
ome Re un se m a rc Im h Immunome Research Burke and Collier, Immunome Res 2014, S:2 ISSN: 1745-7580 DOI: 10.4172/1745-7580.S2.e002 Editorial Open Access Insulitis and Diabetes: A Perspective on Islet Inflammation Susan J Burke and J Jason Collier* Laboratory of Islet Biology and Inflammation Pennington Biomedical Research Center 6400 Perkins Rd., Baton Rouge, LA 70808, USA *Corresponding author: J. Jason Collier, Ph.D, Laboratory of Islet Biology and Inflammation, Pennington Biomedical Research Center, 6400 Perkins Rd., Baton Rouge, LA 70808, USA, Tel: (225) 763-2884; E-mail: [email protected] Received date: 8 April 2014; Accepted date: 12 May 2014; Published date: 30 May 2014 Copyright: © Collier JJ et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract capable of secreting moderate to high amounts of inflammatory mediators, perhaps producing a more sudden onset of diabetes. This Immune cell infiltration into pancreatic islets (termed insulitis) has scenario may explain the reported cases of fulminant diabetes, where been linked with destruction of pancreatic β-cells and thus with onset destruction of β-cells and ensuing diabetes is rapid [5]. of diabetes mellitus. Recently published guidelines for reporting insulitis may generate some deliberation on pancreatic islet Small Quantitative Insulitis with moderate inflammatory Activity: inflammation and a re-examination of the role that immune cells play Fewer leukocytes with sustained, but moderate production of in the process of β-cell death and dysfunction. -
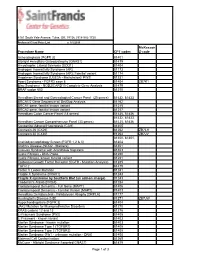
Procedure Name CPT Codes Mckesson Z-Code Achondroplasia
6161 South Yale Avenue, Tulsa, OK, 74136 | 918-502-1720 Preferred Client Price List v. 1/1/2019 McKesson Procedure Name CPT codes Z-code Achondroplasia {FGFR 3} 81401 Albright Hereditary Osteodystrophy {GNAS1} 81479 Amyotrophic Lateral Sclerosis {SOD1} 81404 Androgen Insensitivity Syndrome {AR} 81173 Androgen Insensitivity Syndrome {AR}; Familial variant 81174 Angelman Syndrome {UBE3A - Methylation}/ PWS 81331 Apert Syndrome - FGFR2 exon 8 81404 ZB7K1 Blau Syndrome - NOD2/CARD15 Complete Gene Analysis 81479 BRAF codon 600 81210 Hereditary Breast and Gynecological Cancer Panel (25 genes) 81432, 81433 BRCA1/2 Gene Sequence w/ Del/Dup Analysis 81162 BRCA1 gene, familial known variant 81215 BRCA2 gene, familial known variant 81217 Hereditary Colon Cancer Panel (18 genes) 81435, 81436 81432, 81433, Hereditary Cancer Comprehensive Panel (33 genes) 81435, 81436 Congenital Adrenal Hyperplasia {CAH} 81405 Connexin 26 {CX26} 81252 ZB7LH Connexin 30 {CX30} 81254 ZB7JV 81400, 81401, Craniodysmorphology Screen {FGFR 1,2 & 3} 81404 Crohn's Disease {NOD2 - Markers} 81401 Crouzon Syndrome with Acanthosis Nigricans 81403 Cystic Fibrosis - DNA Probe 81220 Cystic Fibrosis, known familial variant 81221 Epidermal Growth Factor Receptor {EGFR - Mutation Analysis} 81235 FGFR 2 81479 Factor V Leiden Mutation 81241 Fragile X Syndrome {FRAX1} 81243 Fragile X syndrome by Southern Blot (an add-on charge) 81243 Friederich's Ataxia {FRDA} 81284 Frontotemporal Dementia - Full Gene (MAPT) 81406 Frontotemporal Dementia - Familial Variant (MAPT) 81403 Hereditary Dentatorubral