PTEN Mutations the PTEN Hamartoma Tumor Synd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Unusual Case of Cowden Syndrome Associated With
Pistorius et al. Hereditary Cancer in Clinical Practice (2016) 14:11 DOI 10.1186/s13053-016-0051-8 CASE REPORT Open Access An unusual case of Cowden syndrome associated with ganglioneuromatous polyposis Steffen Pistorius1,6*†, Barbara Klink2,7*†, Jessica Pablik3, Andreas Rump2, Daniela Aust3,7, Marlene Garzarolli4, Evelin Schröck2,7 and Hans K. Schackert5,6,7 Abstract Background: Ganglioneuromatous polyposis (GP) is a very rare disorder which may be associated with other clinical manifestations and syndromes, such as Cowden syndrome, multiple endocrine neoplasia (MEN) type II and neurofibromatosis (NF) 1. The risk for malignant transformation of ganglioneuromas is unknown, and the combination of GP with colon cancer has been only very seldom reported. Methods and results: We report the case of a 60-year old male patient with adenocarcinoma, adenomas and lipomas of the colon and multiple gastroduodenal lesions combined with generalised lipomatosis and macrocephaly. Based on the initial endoscopic and histological findings, a (restorative) proctocolectomy was recommended but declined by the patient. Instead, a colectomy was performed. The histological examination revealed an unforeseen GP in addition to the colon cancer. Extensive molecular diagnostics allowed for the differential diagnosis of the causes of the clinical manifestations, and the clinical suspicion of Cowden syndrome could not be confirmed using Sanger Sequencing and MLPA for the analysis of PTEN. Finally, a pathogenic germline mutation in PTEN (heterozygous stop mutation in exon 2: NM_000314 (PTEN):c.138C > A; p.Tyr46*) could be detected by next-generation sequencing (NGS), confirming an unusual presentation of Cowden syndrome with GP. Conclusions: Cowden syndrome should be considered in cases of GP with extracolonic manifestation and verified by combined clinical and molecular diagnostics. -

Blueprint Genetics Comprehensive Growth Disorders / Skeletal
Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel Test code: MA4301 Is a 374 gene panel that includes assessment of non-coding variants. This panel covers the majority of the genes listed in the Nosology 2015 (PMID: 26394607) and all genes in our Malformation category that cause growth retardation, short stature or skeletal dysplasia and is therefore a powerful diagnostic tool. It is ideal for patients suspected to have a syndromic or an isolated growth disorder or a skeletal dysplasia. About Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders This panel covers a broad spectrum of diseases associated with growth retardation, short stature or skeletal dysplasia. Many of these conditions have overlapping features which can make clinical diagnosis a challenge. Genetic diagnostics is therefore the most efficient way to subtype the diseases and enable individualized treatment and management decisions. Moreover, detection of causative mutations establishes the mode of inheritance in the family which is essential for informed genetic counseling. For additional information regarding the conditions tested on this panel, please refer to the National Organization for Rare Disorders and / or GeneReviews. Availability 4 weeks Gene Set Description Genes in the Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ACAN# Spondyloepimetaphyseal dysplasia, aggrecan type, AD/AR 20 56 Spondyloepiphyseal dysplasia, Kimberley -

Blueprint Genetics Comprehensive Skeletal Dysplasias and Disorders
Comprehensive Skeletal Dysplasias and Disorders Panel Test code: MA3301 Is a 251 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of disorders involving the skeletal system. About Comprehensive Skeletal Dysplasias and Disorders This panel covers a broad spectrum of skeletal disorders including common and rare skeletal dysplasias (eg. achondroplasia, COL2A1 related dysplasias, diastrophic dysplasia, various types of spondylo-metaphyseal dysplasias), various ciliopathies with skeletal involvement (eg. short rib-polydactylies, asphyxiating thoracic dysplasia dysplasias and Ellis-van Creveld syndrome), various subtypes of osteogenesis imperfecta, campomelic dysplasia, slender bone dysplasias, dysplasias with multiple joint dislocations, chondrodysplasia punctata group of disorders, neonatal osteosclerotic dysplasias, osteopetrosis and related disorders, abnormal mineralization group of disorders (eg hypopohosphatasia), osteolysis group of disorders, disorders with disorganized development of skeletal components, overgrowth syndromes with skeletal involvement, craniosynostosis syndromes, dysostoses with predominant craniofacial involvement, dysostoses with predominant vertebral involvement, patellar dysostoses, brachydactylies, some disorders with limb hypoplasia-reduction defects, ectrodactyly with and without other manifestations, polydactyly-syndactyly-triphalangism group of disorders, and disorders with defects in joint formation and synostoses. Availability 4 weeks Gene Set Description -

Cowden Syndrome
Update No.4 - 2017 Cowden syndrome Cowden syndrome is a rare inherited multiple hamartoma syndrome with a reported, but likely underestimated, prevalence of 1 in 200,000. Affected individuals usually have macrocephaly and develop benign mucocutaneous lesions (trichilemmomas, papillomatous papules and acral keratosis) by early adulthood. The clinical diagnosis of Cowden syndrome is complex and based on diagnostic criteria divided into 3 categories (pathognomonic, major and minor). Gastrointestinal manifestations are common and can be suggestive of Cowden syndrome in a patient with other manifestations of the disease. Genetics: Autosomal dominant inheritance. Up to 85% of cases are caused by mutations in the PTEN gene on chromosome 10 or its promoter. Mutation of the gene and altered protein product leads to unchecked cellular proliferation. Gastrointestinal tract manifestations: More recent studies suggest that 75-95% of patients with Cowden syndrome will have gastrointestinal manifestations. These can include: Oesophageal glycogenic acanthosis, which often presents as small white lesions endoscopically Multiple hamartomatous polyps of the GI tract Small sessile hamartomatous polyps of the large bowel frequently containing adipose tissue and lymphoid follicles (Fig. 1) Ganglioneuroma (Fig. 2), lipoma, and fibrolipoma of the large bowel Inflammatory/hyperplastic polyps of the stomach Increased risk of adenomas in the large bowel Fig. 1 Hamartomatous polyp Fig. 2 Ganglioneuroma Malignant risk: It is important to recognise Cowden syndrome -

Eponyms Related to Genetic Disorders Associated with Gingival Enlargement; Part I
Historical Article DOI: 10.7241/ourd.20144.114 EPONYMS RELATED TO GENETIC DISORDERS ASSOCIATED WITH GINGIVAL ENLARGEMENT; PART I Ahmad Al Aboud1, Nora Mohammed Al-Aboud2, Hanan Barnawi3, Ahlam Al Hakami3 1Dermatology Department, King Abdullah Medical City, Makkah, Saudi Arabia 2College of Applied Sciences, Umm Al-Qura University, Makkah, Saudi-Arabia 3 Source of Support: Public Health Department, King Faisal hospital, Makkah, Saudi Arabia Nil Competing Interests: None Corresponding author: Dr. Ahmad Al Aboud [email protected] Our Dermatol Online. 2014; 4(5): 439-441 Date of submission: 27.05.2013 / acceptance: 14.07.2014 Cite this article: Al Aboud A, Al-Aboud NM, Barnawi H, Al Hakami A: Eponyms related to genetic disorders associated with gingival enlargement; part I. Our Dermatol Online. 2014; 4(5): 439-441. Gingival enlargement is common among patients and can disorders, vascular disorders and syndromes characterized by be caused by a variety of etiological factors. The most common the presence of characteristic dental abnormalities . reason is poor oral hygiene and high bacterial load that leads Hereditary Gingival Fibromatosis (HGF), represents a to gingival inflammation and enlargement. Other implicated heterogeneous group of disorders characterized by progressive factors include systemic drugs, such as Phenytoin, Nifedipine, enlargement of the gingiva. It manifests itself by an enlarged Verapamil and Cyclosporine. Some enlargements could be gingival tissue covering teeth to various extents. HGF may associated with other conditions such as puberty, pregnancy appear as an isolated entity i.e. as autosomal dominant Gingival or diabetes or be a symptom of a systemic disease (leukemia, Fibromatosis, which has little consequence apart from a cosmetic Wegener’s granulomatosis or sarcoidosis) [1]. -
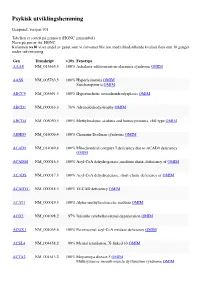
Psykisk Utviklingshemming
Psykisk utviklingshemming Genpanel, versjon v01 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC Kolonnen >x10 viser andel av genet som vi forventer blir lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering Gen Transkript >10x Fenotype AAAS NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AASS NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCC9 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 NM_000033.3 76% Adrenoleukodystrophy OMIM ABCD4 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 NM_014049.4 100% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL NM_000018.3 100% VLCAD deficiency OMIM ACAT1 NM_000019.3 100% Alpha-methylacetoacetic aciduria OMIM ACO2 NM_001098.2 97% Infantile cerebellar-retinal degeneration OMIM ACOX1 NM_004035.6 100% Peroxisomal acyl-CoA oxidase deficiency OMIM ACSL4 NM_004458.2 99% Mental retardation, X-linked 63 OMIM ACTA2 NM_001613.2 100% Moyamoya disease 5 OMIM Multisystemic smooth muscle dysfunction syndrome OMIM Gen Transkript >10x Fenotype ACTB NM_001101.3 100% ?Dystonia, juvenile-onset OMIM Baraitser-Winter syndrome 1 OMIM ACTG1 NM_001614.3 100% Baraitser-Winter syndrome 2 OMIM Deafness, autosomal dominant 20/26 OMIM ACVR1 NM_001105.4 100% Fibrodysplasia ossificans -

Cowden Syndrome
Page 1 of 2 Cowden Syndrome Cowden syndrome (CS) is part of the PTEN hamartoma tumour syndrome, which also includes Bannayan-Riley-Ruvalcaba syndrome (BRRS), PTEN -related Proteus syndrome (PS), and Proteus-like syndrome. This syndrome is associated with germline PTEN gene mutations that are inherited in an autosomal dominant manner. CS is a multi-system condition characterized by hamartomatous overgrowth of tissues. It is associated with a high risk for benign and malignant tumors of the thyroid, breast, and endometrium. Affected individuals usually have macrocephaly as well as specific skin findings (trichilemmomas and papillomatous papules) identified by their late 20s. Referral Criteria • family member with a confirmed PTEN gene mutation – refer for carrier testing • consider referral for PTEN assessment if personal and/or family history includes the following: o breast cancer, epithelial thyroid (follicular or other non-medullary) cancer, endometrial carcinoma AND skin findings: trichilemmomas, papillomatous papules (oral, facial), acral keratosis, palmoplantar keratosis macrocephaly (occipital frontal circumference ≥ 97th percentile) o Lhermitte-Duclos disease (cerebellar dysplastic gangliocytoma) • other features that have been associated with Cowden syndrome include: thyroid lesions (e.g., adenoma, multinodular goiter), intellectual disability, hamartomatous intestinal polyps, fibrocystic disease of the breast, lipomas, fibromas, renal cell carcinoma, other genitourinary tumors, genitourinary malformation, uterine fibroids. Estimated Lifetime Cancer Risks for PTEN mutation carriers breast cancer 85% thyroid cancer (usually follicular) 35% endometrial cancer: 28% renal cancer 34% colon cancer 9% melanoma 6% This document is provided as a general resource and is not meant to replace hereditary cancer risk assessment. www.bccancer.bc.ca/health-professionals/clinical-resources/hereditary-cancer for Referral Form or call 604-877-6000, local 672198 with questions. -

Comprehensive Inherited Cancer Precision Panel Overview
Comprehensive Inherited Cancer Precision Panel Overview Hereditary cancer syndromes are encountered in all medical specialties. Although they account for about 5% of all malignancies, it is of special importance to identify these patients because, unlike patients with sporadic cancers, they require special, long-term care as their predisposition can cause them to develop certain tumors at a relatively early age. These cancers can arise in the lungs, kidneys, liver, pancreas, skin, eyes, heart. Most hereditary cancers are associated with a “germline mutation” that will be present in every cell of the human body. Identification of patients at risk of inherited cancer susceptibility is dependent upon the ability to characterize genes and alterations associated with increased cancer risk as well as gathering a detailed personal and family history aiding in the identification of the mode of inheritance as well as other family members at risk of suffering from this susceptibility. Most hereditary cancer syndromes follow an autosomal dominant inheritance, and the penetrance is high. The Igenomix Comprehensive Inherited Cancer Precision Panel provides a comprehensive analysis of the most common hereditary cancer syndromes using next-generation sequencing (NGS) to fully understand the spectrum of relevant cancer predisposition genes. Indications The Igenomix Comprehensive Inherited Caner Precision Panel is indicated as a screening and diagnostic test in those cases where there are: ‐ Multiple relatives on the same side of the family with the same or related forms of cancer ‐ Cancer at an early age ‐ Early presentation of an aggressive cancer type ‐ Multiple primary cancers in an individual 1 Clinical Utility The clinical utility of this panel is: ‐ Early and accurate genetic diagnosis allowing the most appropriate clinical management of a patient with personal or family history suggestive of a hereditary cancer syndrome. -

Genetic Testing for Neurologic Disorders MP9497
Coverage of any medical intervention discussed in a Dean Health Plan medical policy is subject to the limitations and exclusions outlined in the member's benefit certificate or policy and to applicable state and/or federal laws. Genetic Testing for Neurologic Disorders MP9497 Covered Service: Yes Prior Authorization Required: Yes Additional Genetic testing is covered for a Dean Health Plan member if Information: the test results provide a direct medical benefit or guides reproductive decision-making for the Dean Health Plan member. See Genetic Testing MP9012 for additional information. Pre- and post-test genetic counseling is required for any individual undergoing genetic testing for neurological disorders. Pre and post-genetic counseling is not required for ASO members. Please reference the ASO Summary Plan Document (SPD). A first-degree relative is defined as an individual’s parents, full siblings, and children. A second-degree relative is defined as an individual’s grandparents, grandchildren, aunts, uncles, nephews, nieces and half-siblings. *Axonal neuropathy indicates etiology is related to diabetes, toxic medications, or thyroid disease. Reproductive carrier screening (prenatal testing) does not require prior authorization and is addressed per MP9477 Medicare Policy: Prior authorization is dependent on the member’s Medicare coverage. Prior authorization is not required for Medicare Cost products (Dean Care Gold) and Medicare Supplement (Select) when this service is provided by participating providers. Prior authorization is required if a member has Medicare primary and Dean Health Plan secondary coverage. This policy is not applicable to our Medicare Replacement products. BadgerCare Plus Dean Health Plan covers when BadgerCare Plus also covers Policy: the benefit. -

Cowden Syndrome Is a Risk Factor for Multiple Neoplasm: a Case Report Sofia Miguelote1* , Roberto Silva2,3, J
Miguelote et al. World Journal of Surgical Oncology (2020) 18:211 https://doi.org/10.1186/s12957-020-01971-z CASE REPORT Open Access Cowden syndrome is a risk factor for multiple neoplasm: a case report Sofia Miguelote1* , Roberto Silva2,3, J. L. Fougo2,4, L. E. Barbosa1,2,5 and J. P. Araújo Teixeira1,2,5 Abstract Background: Cowden’s syndrome is an autosomal dominant disease with variable penetrance, involving the tumor suppressor phosphatase and tension homolog gene, located on chromosome 10q22-23, responsible for cell proliferation, migration, and cellular apoptosis. Its clinical presentation encompasses mucocutaneous lesions, which are present around 99% of the time; macrocephaly; and cognitive impairment, and it precedes the appearance of neoplasms such as thyroid carcinoma, breast cancer, among others. In addition to these malformations, arteriovenous malformations of the brain and spine, endocrine abnormalities, skeletal defects, and cardiopulmonary lesions may also be found. The relevance of the case is due to the fact that, through a certain phenotype, the patient’s genotype can be inferred and thus followed up closely. Case representation: The clinical case concerns a 28-year-old Caucasian and Portuguese woman with palmar pits, macrocephaly, and cognitive impairment. She was diagnosed with papillary thyroid carcinoma at 22 years of age and proposed total thyroidectomy. At age 27, a pregnancy was diagnosed with a Breast Imaging-Reporting and Data System 2-rated breast lump. After the histological verification, it was concluded that it was a high metastatic breast sarcoma, opting for palliative mastectomy. A genetic evaluation confirmed alteration in the phosphatase and tension homolog gene, confirming Cowden’s syndrome. -
Kodachrome Session
2/14/2017 F001 Oral Signs of Genetic Disease DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Jennifer L. Hand, MD Jennifer L. Hand MD F001- Oral Signs of Genetic Disease Associate Professor of Dermatology, 10:10 AM – 10:30 AM Clinical Genomics, and Pediatrics DISCLOSURES Mayo Clinic, Rochester, MN I do not have any relevant relationships with industry. Objectives Syndromes Connective Tissue Dysplasia • Marfan Syndrome • Diagnose oral signs of genetic disease more Neoplastic accurately • Peutz- Jegher • Ehlers-Danlos syndrome • Familial Adenomatous • Osteogenesis Imperfecta • Recognize benign skin findings that indicate Polyposis (FAP) • Dentinogenesis imperfecta an increased risk for systemic disease • MEN2B Ectodermal dysplasia • Obtain a targeted family history for genetic • Cowden syndrome • Hypohydrotic Ectodermal syndromes with oral features dysplasia • Carney Complex • Incontinentia Pigmenti Which associated skin change is most likely? A) Striae B) Mucosal neuromas C) Syringomas D) Basal cell nevi E) Mucous cysts 1 2/14/2017 Which associated skin change is Marfan syndrome most likely? • Autosomal dominant, variable expressivity A) Striae • 1:5,000 persons B) Mucosal neuromas • 25% new (de novo) mutations C) Syringomas • Affects skeletal, ocular and cardiovascular systems D) Basal cell nevi • Potentially fatal; may not be evident until adolescence E) Mucous cysts Ghent Systemic Score Marfan.org Marfan syndrome Score > or = 7 is significant. Marfan Syndrome • Fibrillin-1 (FBN1) mutation Feature Value Feature Value • Extracellular matrix -

Whole Exome Sequencing Gene Package Oncogenetics, Version 2.2, 31-1-2020
Whole Exome Sequencing Gene package Oncogenetics, version 2.2, 31-1-2020 Technical information DNA was enriched using Agilent SureSelect DNA + SureSelect OneSeq 300kb CNV Backbone + Human All Exon V7 capture and paired-end sequenced on the Illumina platform (outsourced). The aim is to obtain 10 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate and non-unique reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA-MEM algorithm (reference: http://bio-bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A2ML1 No OMIM phenotype 610627 44 100 97 80 ACD ?Dyskeratosis congenita, autosomal dominant 6, 616553 609377 92 100 100 100 ?Dyskeratosis congenita, autosomal recessive 7, 616553 AIP Pituitary adenoma, growth hormone-secreting,