Renal Cellular and Tissue Specializations in the Bottlenose Dolphin (Tursiops Truncatus) and Beluga Whale (Delphinapterus Leucas)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cetacean Records Along a Coastal-Offshore Gradient in the Vitória
DOI: http://dx.doi.org/10.1590/1519-6984.21812 Cetacean records along a coastal-offshore gradient in the Vitória- Trindade Chain, western South Atlantic Ocean Wedekin, LL.a*, Rossi-Santos, MR.a, Baracho, C.a, Cypriano-Souza, AL.a,b and Simões-Lopes, PC.c aInstituto Baleia Jubarte, Rua Barão do Rio Branco, 125, CEP 45900-000, Caravelas, BA, Brazil bLaboratório de Biologia Genômica e Molecular, Faculdade de Biociências, Pontifícia Universidade Católica do Rio Grande do Sul – PUCRS, Avenida Ipiranga, 6681, CEP 90619-900, Porto Alegre, RS, Brazil cLaboratório de Mamíferos Aquáticos – LAMAQ, Departamento de Ecologia e Zoologia, Universidade Federal de Santa Catarina – UFSC, Campus Universitário, CP 5102, Trindade, CEP 88040-970, Florianópolis, SC, Brazil *e-mail: [email protected] Received: November 2, 2012 – Accepted: December 27, 2012 – Distributed: February 28, 2014 (With 2 figures) Abstract Oceanic waters are difficult to assess, and there are many gaps in knowledge regarding cetacean occurrence. To fill some of these gaps, this article provides important cetacean records obtained in the winter of 2010 during a dedicated expedition to collect visual and acoustic information in the Vitória-Trindade seamounts. We observed 19 groups of cetaceans along a 1300-km search trajectory, with six species being identified: the humpback whale Megaptera( novaeangliae, N = 9 groups), the fin whale (Balaenoptera physalus, N = 1), the Antarctic minke whale (Balaenoptera bonaerensis, N = 1), the rough-toothed dolphin (Steno bredanensis, N = 1), the bottlenose dolphin (Tursiops truncatus, N = 2), and the killer whale (Orcinus orca, N = 1). Most humpback whale groups (N = 7; 78%) were observed in the Vitória-Trindade seamounts, especially the mounts close to the Abrolhos Bank. -

FC Inshore Cetacean Species Identification
Falklands Conservation PO BOX 26, Falkland Islands, FIQQ 1ZZ +500 22247 [email protected] www.falklandsconservation.com FC Inshore Cetacean Species Identification Introduction This guide outlines the key features that can be used to distinguish between the six most common cetacean species that inhabit Falklands' waters. A number of additional cetacean species may occasionally be seen in coastal waters, for example the fin whale (Balaenoptera physalus), the humpback whale (Megaptera novaeangliae), the long-finned pilot whale (Globicephala melas) and the dusky dolphin (Lagenorhynchus obscurus). A full list of the species that have been documented to date around the Falklands can be found in Appendix 1. Note that many of these are typical of deeper, oceanic waters, and are unlikely to be encountered along the coast. The six species (or seven species, including two species of minke whale) described in this document are observed regularly in shallow, nearshore waters, and are the focus of this identification guide. Questions and further information For any questions about species identification then please contact the Cetaceans Project Officer Caroline Weir who will be happy to help you try and identify your sighting: Tel: 22247 Email: [email protected] Useful identification guides If you wish to learn more about the identification features of various species, some comprehensive field guides (which include all cetacean species globally) include: Handbook of Whales, Dolphins and Porpoises by Mark Carwardine. 2019. Marine Mammals of the World: A Comprehensive Guide to Their Identification by Thomas A. Jefferson, Marc A. Webber, and Robert L. Pitman. 2015. Whales, Dolphins and Seals: A Field Guide to the Marine Mammals of the World by Hadoram Shirihai and Brett Jarrett. -

Z Dolphin Rescue
z Annual newsletter of the Blue World Institute for Marine Research and Conservation 2007. bottlenose dolphin can weigh up to 350kg which is normally supported by the water. The animal Dolphin rescue should be supported in a stretcher where possible and not left on any hard surfaces, this may dam- age the fragile bone structure of the ribs and the animal’s internal organs. Untrained help therefore from concerned citizens, although understand- able, should be avoided. Veterinary help should applied only by those individuals that have been trained on an internationally recognised cetacean medical course. The serious issue related to the transmission of diseases in cetacean species and populations and to their handling has been dis- cussed in detail at the 59th Annual Meeting of the Scientific Committee of the International Whaling Commission, which Croatia has recently joined. Blue World is a professional scientific organisation regularly participating in workshops and meetings organised by European and world experts trained in cetacean veterinary medicine. The above rec- ommendations follow the international standards of present knowledge on cetacean and dolphin Dolphin rescue is an unusually hard and extremely advice remains the same in this instance: please rescue techniques. In the past, Blue World has complex procedure. Unfortunately it is rarely suc- leave the animals alone, not to catch them or try proposed the creation of a national rescue centre cessful. Any attempt to rescue a whale or dolphin to get in contact with them, not to go into the for endangered and protected marine organisms, should be only carried out by expert personnel sea and swim with them, the best human help to particularly marine turtles and cetaceans, and a specially trained in this procedure. -

The Possible Reasons for Bottlenose Dolphins (Tursiops Truncatus) Participating In
The possible reasons for bottlenose dolphins (Tursiops truncatus) participating in non-predatory aggressive interactions with harbour porpoises (Phocoena phocoena) in Cardigan Bay, Wales Leonora Neale Student ID: 4103778 BSc Zoology Supervised by Dr Francis Gilbert Word count: 5335 Contents Page Page: ABSTRACT……………………………………………………………………….......1 INTRODUCTION…………………………………………………..............................3 METHODS………………………………………………………………....................9 Study species………………………………………………………………......9 Study area………………………………………………………………….…10 Methods of data collection………………………………………...................10 Methods of data analysis………………………………………......................13 RESULTS………………………………………………………………………........14 Geographical distribution……………………………………………….......14 Object-oriented play……………………………………………………........15 DISCUSSION………………………………………………………………….…….18 Geographical distribution……………………………………………………18 Object-oriented play…………………………………………………….........18 Diet………………………………………………………………...................21 CONCLUSION………………………………………………………………………23 ACKNOWLEDGEMENTS………………………………………………………….25 REFERENCES………………………………………………………………………26 APPENDIX……………………………………………………………………..........33 CBMWC sightings………………………...………………………………….…33 CBMWC sightings form guide………………...………………………….….34 CBMWC excel spreadsheet equations……………………………………......35 Abstract Between 1991 and 2011, 137 harbour porpoises (Phocoena phocoena) died as a result of attacks by bottlenose dolphins (Tursiops truncatus) in Cardigan Bay. The suggested reasons for these non-predatory aggressive interactions -

Tursiops Truncatus)
Bottlenose Dolphin (Tursiops truncatus) Image from UNCW Marine Mammal Program Image from UNCW Marine Mammal Program Taken under NOAA scientific permit #948-1692-00 Taken under NOAA scientific permit #948-1692-00 Species Description: - Body coloration ranges from light grey to black dorsally - Robust body and moderately falcate dorsal fin and laterally, with a lighter colored belly - Sharp demarcation between melon and short rostrum - Body size and appendage shape varies across - Pectoral flipper’s leading edge convex, pointed tips geographic regions - Flukes concavely curved along trailing margin and - Average adult length is 6-12 ft (2-3.8 m) notched in center - Average adult weight is 300-1400 lbs (135-635 kg) Behavior: Reproduction: - Fast, efficient swimmers - Lifespan for males - 40-45 yrs, females - over 50 yrs - Cruise at speeds of 1.4 to 3.1 meters per second (3.1-6.9 - Sexual maturity for males - 9-14 yrs, females - 5-13 yrs miles per hour) - Gestation period approximately 12 months - Coastal form found in groups of 2-15 individuals - Calving season usually occurs in warmer months - Groups types include: female bands/nursery, subadult, and male pairs Diet: - Fish Threats / Conservation: Conservation/ Threats: - Benthic invertebrates - Not endangered - Pelagic fish and squids - Protected under the Marine Mammal Protection Act in - Feeding strategies include “fish whacking”, “kerplunking”, United States “crater” feeding, and herding - Bycatch from gillnets, seines, trawls and fishing gear - In 2013-14 experienced a Morbillivirus -

Baiji Genomes Reveal Low Genetic Variability and New Insights Into Secondary Aquatic Adaptations
ARTICLE Received 13 Apr 2013 | Accepted 3 Oct 2013 | Published 29 Oct 2013 DOI: 10.1038/ncomms3708 OPEN Baiji genomes reveal low genetic variability and new insights into secondary aquatic adaptations Xuming Zhou1,2,*, Fengming Sun3,*, Shixia Xu1,*, Guangyi Fan3, Kangli Zhu1, Xin Liu3, Yuan Chen1, Chengcheng Shi3, Yunxia Yang1, Zhiyong Huang3, Jing Chen3, Haolong Hou3, Xuejiang Guo4, Wenbin Chen3, Yuefeng Chen1, Xiaohong Wang1, Tian Lv3, Dan Yang1, Jiajian Zhou3, Bangqing Huang3, Zhengfei Wang1, Wei Zhao3, Ran Tian1, Zhiqiang Xiong3, Junxiao Xu1, Xinming Liang3, Bingyao Chen1, Weiqing Liu3, Junyi Wang3, Shengkai Pan3, Xiaodong Fang3, Ming Li2, Fuwen Wei2, Xun Xu3, Kaiya Zhou1, Jun Wang3,5,6 & Guang Yang1 The baiji, or Yangtze River dolphin (Lipotes vexillifer), is a flagship species for the conservation of aquatic animals and ecosystems in the Yangtze River of China; however, this species has now been recognized as functionally extinct. Here we report a high-quality draft genome and three re-sequenced genomes of L. vexillifer using Illumina short-read sequencing technology. Comparative genomic analyses reveal that cetaceans have a slow molecular clock and molecular adaptations to their aquatic lifestyle. We also find a significantly lower number of heterozygous single nucleotide polymorphisms in the baiji compared to all other mammalian genomes reported thus far. A reconstruction of the demographic history of the baiji indicates that a bottleneck occurred near the end of the last deglaciation, a time coinciding with a rapid decrease in temperature and the rise of eustatic sea level. 1 Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210023, China. -

Review of Small Cetaceans. Distribution, Behaviour, Migration and Threats
Review of Small Cetaceans Distribution, Behaviour, Migration and Threats by Boris M. Culik Illustrations by Maurizio Wurtz, Artescienza Marine Mammal Action Plan / Regional Seas Reports and Studies no. 177 Published by United Nations Environment Programme (UNEP) and the Secretariat of the Convention on the Conservation of Migratory Species of Wild Animals (CMS). Review of Small Cetaceans. Distribution, Behaviour, Migration and Threats. 2004. Compiled for CMS by Boris M. Culik. Illustrations by Maurizio Wurtz, Artescienza. UNEP / CMS Secretariat, Bonn, Germany. 343 pages. Marine Mammal Action Plan / Regional Seas Reports and Studies no. 177 Produced by CMS Secretariat, Bonn, Germany in collaboration with UNEP Coordination team Marco Barbieri, Veronika Lenarz, Laura Meszaros, Hanneke Van Lavieren Editing Rüdiger Strempel Design Karina Waedt The author Boris M. Culik is associate Professor The drawings stem from Prof. Maurizio of Marine Zoology at the Leibnitz Institute of Wurtz, Dept. of Biology at Genova Univer- Marine Sciences at Kiel University (IFM-GEOMAR) sity and illustrator/artist at Artescienza. and works free-lance as a marine biologist. Contact address: Contact address: Prof. Dr. Boris Culik Prof. Maurizio Wurtz F3: Forschung / Fakten / Fantasie Dept. of Biology, Genova University Am Reff 1 Viale Benedetto XV, 5 24226 Heikendorf, Germany 16132 Genova, Italy Email: [email protected] Email: [email protected] www.fh3.de www.artescienza.org © 2004 United Nations Environment Programme (UNEP) / Convention on Migratory Species (CMS). This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. -

Status and Conservation of Cetaceans in the Adriatic Sea
United Nations Environment Programme MEDITERRANEAN ACTION PLAN Regional Activity Centre for Specially Protected Areas STATUS AND CONSERVATION OF CETACEANS IN THE ADRIATIC SEA Draft internal report Draft to be used only to support the preparation of documents for the “Mediterranean Regional Workshop to Facilitate the Description of Ecologically or Biologically Significant Marine Areas. Malaga, Spain, 7-11 April 2014” March 2014 Draft internal report not for distribution This report should be quoted as: UNEP-MAP-RAC/SPA. 2014. Status and Conservation of Cetaceans in the Adriatic Sea. By D. Holcer, C.M. Fortuna & P. C. Mackelworth. Draft internal report for the purposes of the Mediterranean Regional Workshop to Facilitate the Description of Ecologically or Biologically Significant Marine Areas, Malaga, Spain, 7-11 April 2014. Contents 1 Context .......................................................................................................................................... 5 2 The Adriatic Sea ............................................................................................................................. 7 3 Cetacean species in the Adriatic Sea ............................................................................................... 8 3.1 The common bottlenose dolphin (Tursiops truncatus) .................................................................... 8 3.1.1 Distribution and abundance ...................................................................................................... 8 3.1.1.1 Trends in -

Cetaceans of the Red Sea - CMS Technical Series Publication No
UNEP / CMS Secretariat UN Campus Platz der Vereinten Nationen 1 D-53113 Bonn Germany Tel: (+49) 228 815 24 01 / 02 Fax: (+49) 228 815 24 49 E-mail: [email protected] www.cms.int CETACEANS OF THE RED SEA Cetaceans of the Red Sea - CMS Technical Series Publication No. 33 No. Publication Series Technical Sea - CMS Cetaceans of the Red CMS Technical Series Publication No. 33 UNEP promotes N environmentally sound practices globally and in its own activities. This publication is printed on FSC paper, that is W produced using environmentally friendly practices and is FSC certified. Our distribution policy aims to reduce UNEP‘s carbon footprint. E | Cetaceans of the Red Sea - CMS Technical Series No. 33 MF Cetaceans of the Red Sea - CMS Technical Series No. 33 | 1 Published by the Secretariat of the Convention on the Conservation of Migratory Species of Wild Animals Recommended citation: Notarbartolo di Sciara G., Kerem D., Smeenk C., Rudolph P., Cesario A., Costa M., Elasar M., Feingold D., Fumagalli M., Goffman O., Hadar N., Mebrathu Y.T., Scheinin A. 2017. Cetaceans of the Red Sea. CMS Technical Series 33, 86 p. Prepared by: UNEP/CMS Secretariat Editors: Giuseppe Notarbartolo di Sciara*, Dan Kerem, Peter Rudolph & Chris Smeenk Authors: Amina Cesario1, Marina Costa1, Mia Elasar2, Daphna Feingold2, Maddalena Fumagalli1, 3 Oz Goffman2, 4, Nir Hadar2, Dan Kerem2, 4, Yohannes T. Mebrahtu5, Giuseppe Notarbartolo di Sciara1, Peter Rudolph6, Aviad Scheinin2, 7, Chris Smeenk8 1 Tethys Research Institute, Viale G.B. Gadio 2, 20121 Milano, Italy 2 Israel Marine Mammal Research and Assistance Center (IMMRAC), Mt. -
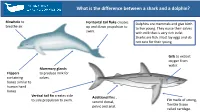
What Is the Difference Between a Shark and a Dolphin?
What is The Difference BetweenWhat a is the difference between a shark and a dolphin? Shark and a Dolphin? Blowhole to Horizontal tail fluke creates Dolphins are mammals and give birth breathe air. up and down propulsion to to live young. They nurse their calves swim. with milk that is very rich in fat. Sharks are fish. Most lay eggs and do not care for their young. Gills to extract oxygen from water. Mammary glands Flippers to produce milk for containing calves. bones similar to human hand bones. Vertical tail fin creates side Additional fins , Fin made of strong, to side propulsion to swim. second dorsal, flexible tissue pelvic and anal. called cartilage. What is The Difference BetweenWhat a is the difference between a whale and a dolphin? Shark and a Dolphin? Two blowholes Baleen Whale Baleen is the bristle like structure to breathe air. in a whale’s upper jaw which it uses to filter small fish or crustaceans from the water. Single blowhole to Many species we call whales breathe air. are more closely related to Throat pleats in some baleen dolphins. Generally scientists whales expand to fill with water Dolphin talk about baleen whales and small fish or crustaceans and and toothed then contract, pushing the water whales. Toothed whales back through the baleen. include sperm whales, beaked whales, and all Teeth on the upper and lower porpoises and dolphins. jaws to grab fish, squid or other Killer whales and pilot prey. They use echolocation to whales are actually dolphins. find their food. What is The Difference BetweenEcholocation a Shark and a Dolphin? Toothed whales (dolphins, porpoises and species like pilot Brain processes Nasal passage whales and killer whales) use echolocation to navigate and find signals to form an contains their food. -

Federal Register/Vol. 84, No. 229/Wednesday, November 27
Federal Register / Vol. 84, No. 229 / Wednesday, November 27, 2019 / Notices 65353 consideration during the meeting, and reports-species-stock#cetaceans---large- Background to ensure transmission to the Committee whales. Section 117 of the MMPA (16 U.S.C. prior to the meeting, comments must be Copies of the Alaska Regional SARs 1361 et seq.) requires NMFS and the received no later than 5:00 p.m. EST on may be requested from Marcia Muto, U.S. Fish and Wildlife Service (FWS) to Monday, December 16, 2019. Comments Alaska Fisheries Science Center, NMFS, prepare stock assessments for each stock received after that date will be 7600 Sand Point Way NE, Seattle, WA of marine mammals occurring in waters distributed to the members but may not 98115–6349. under the jurisdiction of the United be considered at the meeting. Copies of the Atlantic, Gulf of Mexico, States, including the U.S. Exclusive Copies of CINTAC meeting minutes and Caribbean Regional SARs may be Economic Zone. These reports must will be available within 90 days of the requested from Elizabeth Josephson, contain information regarding the meeting. Northeast Fisheries Science Center, 166 distribution and abundance of the stock, Dated: November 14, 2019. Water St., Woods Hole, MA 02543. population growth rates and trends, Devin Horne, Copies of the Pacific Regional SARs estimates of annual human-caused Designated Federal Officer, Office of Energy may be requested from Jim Carretta, mortality and serious injury (M/SI) from and Environmental Industries. Southwest Fisheries Science Center, all sources, descriptions of the fisheries [FR Doc. 2019–25786 Filed 11–26–19; 8:45 am] 8604 La Jolla Shores Drive, La Jolla, CA with which the stock interacts, and the BILLING CODE 3510–DR–P 92037–1508. -

Whale Classification & Food Chains
Whale Classification & Food Chains Student Activity There are two different groups of whales, those that have teeth (called the toothed whales), and those that have no teeth (called the baleen whales). Look Out for These Keywords... Adaptation - a feature of the body that helps an animal survive in its environment Blubber - a thick layer of fat under the skin Crustaceans - large group of animals which includes crabs, prawns and shrimps Predator - an animal that eats other animals Prey - an animal that is hunted, caught and eaten by another animal Baleen Whales Baleen whales are predators that sieve zooplankton including tiny crustaceans such as krill, and small fish from the water using comb-like structures called baleen that are attached to the upper jaw. As they feed, baleen whales gulp large amounts of water and prey. Using their tongues, they push out the water leaving behind krill and fish trapped in their baleen. A Southern right whale is a type of baleen whale. Right whales use their baleen to filter zooplankton at the waters surface. There are 15 species of baleen whales found throughout the world. These include the blue whale, the largest animal to have ever lived. Blue whales grow to 25m and can weigh up to 200tonnes. Blue Whale 25m Humpback whales are another type of baleen whale. There are different populations of humpback whales found in the northern and southern hemispheres of the world. Humpback whales feed in the cold waters of Antarctica and the Arctic during the summer months where their prey, krill and small schooling fish are abundant.