Orca.Cf.Ac.Uk/110865
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parasites of Coral Reef Fish: How Much Do We Know? with a Bibliography of Fish Parasites in New Caledonia
Belg. J. Zool., 140 (Suppl.): 155-190 July 2010 Parasites of coral reef fish: how much do we know? With a bibliography of fish parasites in New Caledonia Jean-Lou Justine (1) UMR 7138 Systématique, Adaptation, Évolution, Muséum National d’Histoire Naturelle, 57, rue Cuvier, F-75321 Paris Cedex 05, France (2) Aquarium des lagons, B.P. 8185, 98807 Nouméa, Nouvelle-Calédonie Corresponding author: Jean-Lou Justine; e-mail: [email protected] ABSTRACT. A compilation of 107 references dealing with fish parasites in New Caledonia permitted the production of a parasite-host list and a host-parasite list. The lists include Turbellaria, Monopisthocotylea, Polyopisthocotylea, Digenea, Cestoda, Nematoda, Copepoda, Isopoda, Acanthocephala and Hirudinea, with 580 host-parasite combinations, corresponding with more than 370 species of parasites. Protozoa are not included. Platyhelminthes are the major group, with 239 species, including 98 monopisthocotylean monogeneans and 105 digeneans. Copepods include 61 records, and nematodes include 41 records. The list of fish recorded with parasites includes 195 species, in which most (ca. 170 species) are coral reef associated, the rest being a few deep-sea, pelagic or freshwater fishes. The serranids, lethrinids and lutjanids are the most commonly represented fish families. Although a list of published records does not provide a reliable estimate of biodiversity because of the important bias in publications being mainly in the domain of interest of the authors, it provides a basis to compare parasite biodiversity with other localities, and especially with other coral reefs. The present list is probably the most complete published account of parasite biodiversity of coral reef fishes. -

AN ASSESSMENT of the KENYAN COASTAL ARTISANAL FISHERY and IMPLICATIONS for the INTRODUCTION of Fads Thesis Submitted in Fulfillm
An Assessment of the Kenyan Coastal Artisanal Fishery and Implications for the Introduction of FADs. Item Type Thesis/Dissertation Authors Mbaru, Emmanuel Kakunde Publisher Rhodes University Download date 24/09/2021 00:29:40 Link to Item http://hdl.handle.net/1834/6844 AN ASSESSMENT OF THE KENYAN COASTAL ARTISANAL FISHERY AND IMPLICATIONS FOR THE INTRODUCTION OF FADs Thesis submitted in fulfillment of the requirements for the degree of MASTER OF SCIENCE of RHODES UNIVERSITY By EMMANUEL KAKUNDE MBARU December 2012 i ABSTRACT The marine fishery in Kenya is predominantly small-scale and artisanal with about 11,000 fishers intensely fishing near shore coastal reefs using minimally selective fishing gears. A large majority (88%) of fishers use outdated equipment such as basket traps, beach seines, hand lines (hook and lines), fence traps, gillnets, spearguns and cast nets. Handmade canoes propelled by paddles (kasia) or sail power are used to access offshore waters, while only a few fishers have motorized boats. Although fishers along this coast know and express the potential of offshore fishing, most of them are disempowered and unable to access any of the largely untapped offshore pelagic resources. Using a unique dataset from four distinct coastal areas: Funzi-Shirazi bay area, Diani-Chale area, Mombasa-Kilifi north coast area and the Malindi-Ungwana bay area, containing species level length frequency catch data from the multi-gear and multi-species fishery, abundance of specific species, gear use comparisons in various regions, catch per unit effort and total catch estimate over a nine year period (2001 – 2009) were evaluated. Despite high diversity in the fishery, five species (Lethrinus lentjan, Siganus sutor, Leptoscarus vaigiensis, Lethrinus harak and Parupeneus macronemus) represented over 75% of the catch. -

Reproductive Biology of Lethrinus Harak on Guam
Reproductive biology of Lethrinus harak on Guam Compiled by Brett M Taylor and Jennifer L McIlwan University of Guam Marine Laboratory Technical Report March 2 Reproduction in Lethrinus harak TABLE OF CONTENTS SUMMARY ................................................................................................ 3 INTRODUCTION ......................................................................................... 4 METHODS ................................................................................................. 5 Processing of gonads ...................................................................................................... 5 Maturation and sex reversal ........................................................................................... 6 Reproductive seasonality ................................................................................................ 6 Reproductive development ............................................................................................ 6 RESULTS .................................................................................................... 6 Ovarian stages ................................................................................................................. 7 Seasonal variability ......................................................................................................... 8 Developmental ontogeny ............................................................................................... 8 DISCUSSION ........................................................................................... -

National Prioritization of Key Vulnerable Reef Fish Species for Fiji, for Targeted Research
National prioritization of key vulnerable reef fish species for Fiji, for targeted research Coral reef fish and invertebrates sold at the Suva market. Photo by: Sangeeta Mangubhai/WCS Introduction The majority of Fiji’s population is coastal and therefore highly reliant on inshore fisheries for their subsistence and local economic needs (Hunt 1999). At least 33 percent of all animal protein consumed in Fiji comes from fish, and subsistence and artisanal fisheries contribute at least US$59.1 million to Fiji’s annual GDP (Gillett 2009). There is growing concerns for the impacts of present day harvesting rates and methods, especially for vulnerable fish and invertebrate species in Fiji. This is resulting in a progressive decline in fish belonging to higher trophic (feeding) groups, a pattern that is termed “fishing down food webs” (Pauly et al. 1998). Coral reef fish vary in their vulnerability to fishing pressure, and how well they can recover, if fishing is stopped or significantly reduced. Recovery potential relates to the rate at which a species can replace the individuals that are lost to natural mortality and to fishing. In general, the medium to larger carnivorous fish high in the food chain are thought to be more vulnerable to fishing (e.g. groupers) requiring in decades to recover, while smaller fish (e.g. herbivores such as rabbitfish) are thought be less vulnerable (Abesamis et al. 2014). Certain life history characteristics of fish species together can be good predictors of vulnerability at the population level to fishing pressure, including: (a) maximum size; (b) body growth rate; (c) lifespan; (d) natural mortality rates; (e) age at maturity; and (f) length at maturity (Abesamis et al. -

Diel Habitat-Use Patterns of Commercially Important Fishes in a Marine Protected Area in the Philippines
Vol. 24: 163–174, 2016 AQUATIC BIOLOGY Published online January 27 doi: 10.3354/ab00646 Aquat Biol OPENPEN ACCESSCCESS Diel habitat-use patterns of commercially important fishes in a marine protected area in the Philippines Kentaro Honda1,4,*, Wilfredo H. Uy2, Darwin I. Baslot2, Allyn Duvin S. Pantallano2, Yohei Nakamura3, Masahiro Nakaoka1 1Akkeshi Marine Station, Field Science Center for Northern Biosphere, Hokkaido University, Aikappu, Akkeshi, Hokkaido 088-1114, Japan 2Institute of Fisheries Research and Development, Mindanao State University at Naawan, 9023 Naawan, Misamis Oriental, the Philippines 3Graduate School of Kuroshio Science, Kochi University, 200 Monobe, Nankoku, Kochi 783-8502, Japan 4Present address: Hokkaido National Fisheries Research Institute, Fisheries Research Agency, 2-2 Nakanoshima, Toyohira-ku, Sapporo, Hokkaido 062-0922, Japan ABSTRACT: The diel habitat-use patterns of commercially important fishes in a small marine protected area (MPA) (0.31 km2) containing coral reef and seagrass habitats were examined by passive acoustic telemetry during 2011 and 2012. The occurrence patterns of the target fishes both inside and outside the MPA were also observed. Thirty individuals from 6 species (20.2 to 41.4 cm fork length) were caught, acoustically tagged and released inside the MPA, and 4 to 210 d of tracking data were then obtained from 28 detected fishes. Lutjanus monostigma, Lethrinus atkin- soni, and Lethrinus obsoletus were found to mostly inhabit the coral reef. The remaining 3 species (Lutjanus argentimaculatus, Lethrinus harak, and Siganus guttatus) utilized both coral and seagrass habitats but showed different patterns: Lutjanus argentimaculatus visited seagrass only at night; Lethrinus harak occurred in the coral reef more at night than in the day, showing the opposite pattern in seagrass; and S. -

Fish Movement in the Red Sea and Implications for Marine Protected Area Design
Fish Movement in the Red Sea and Implications for Marine Protected Area Design Thesis by Irene Antonina Salinas Akhmadeeva In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology Thuwal, Kingdom of Saudi Arabia April, 2021 2 EXAMINATION COMMITTEE PAGE The thesis of Irene Antonina Salinas Akhmadeeva is approved by the examination committee. Committee Chairperson: Prof. Michael L. Berumen Committee Co-Chair: Dr. Alison Green Committee Members: Dr. Darren Coker, Prof. Rusty Brainard 3 COPYRIGHT © April 2021 Irene Antonina Salinas Akhmadeeva All Rights Reserved 4 ABSTRACT Fish Movement in the Red Sea and Implications for Marine Protected Area Design Irene Antonina Salinas Akhmadeeva The Red Sea is valued for its biodiversity and the livelihoods it provides for many. It now faces overfishing, habitat degradation, and anthropogenic induced climate-change. Marine Protected Areas (MPAs) became a powerful management tool to protect vulnerable species and ecosystems, re-establish their balance, and enhance marine populations. For this, they need to be well designed and managed. There are 15 designated MPAs in the Red Sea but their level of enforcement is unclear. To design an MPA it is necessary to know if it will protect species of interest by considering their movement needs. In this thesis I aim at understanding fish movement in the Red Sea, specifically home range (HR) to inform MPA size designation. With not much empirical data available on HR for Red Sea fish, I used a Machine Learning (ML) classification model, trained with empirical literature HR measurements with Maximum Total Length (L Max), Aspect Ratio (AR) of the caudal fin, and Trophic Level as predictor variables. -

Coastal Marine Fish Biodiversity Along the Western Coast of India.Journal of Threatened Taxa 5(1): 3574––3579; Doi:10.11609/Jott
Journal of Threatened Taxa | www.threatenedtaxa.org | January 2013 | 5(1): 3574–3579 Note Coastal marine fish biodiversity along the March 2002 (n=7 sites and 30 hours western coast of India sampling). Similar habitat was examined at Natrani Island, offshore Robert D. Sluka of Murdeshwar, Karnataka State ISSN and Grand Island offshore of Panaji, Online 0974-7907 Chiltern Gateway Project, A Rocha UK, 18-19 Avenue Rd, Southall, Middlesex Print 0974-7893 UB1 3BL, United Kingdom Goa State (Image 1), September– [email protected] October 2002 (n = all surveys sum to oPEN ACCESS 8 and 13 hours of observation time, respectively, for each of these two sites). The habitat The commercial fish fauna of India has been studied surveyed in Kerala and Tamil Nadu (depth range 3–30 m) extensively (James et al. 1994, 1996). However, little was rocky with low coral cover (<1%) (Sluka et al. 2012). is known about noncommercial fishes (Kuthalingam The most abundant benthic colonizers were fine turf et al. 1973, 1979; Sreenivasan & Lazarus 1973; James algae, encrusting sponges, barnacles and mussels. At et al. 1988; Murty 2002). Underwater visual censuses Muttom, three types of habitats were surveyed and each have been reported from Lakshadweep (Anand & Pillai considered a separate site: (i) shallow (<10m), inshore 2002, 2005), the Andaman and Nicobar Islands (Madhu (<500m from shore), dead coral reef; (ii) offshore rocky & Madhu 2007; Rajaram & Nedumaran 2009) and from islands (15m depth); and (iii) submerged rocky habitat islands off the central western coast states of Goa and (30m depth). The habitat in western coast off Karnataka Karnataka (Sluka & Lazarus 2004, 2005, 2006, 2009, and Goa (depth range 2–12 m) was also rocky, but with 2010; Zacharia et al. -

Aspects of the Biology and Feeding Ecology of the Orbiculate Cardinal Fish Sphaeramia Orbicularis (Cuvier, 1828) (Teleostei : Apogonidae) in a Kenyan Mangrove Forest
Biol Jaarb. Dodonaea, 66, 1998 (1999), 134-145 ASPECTS OF THE BIOLOGY AND FEEDING ECOLOGY OF THE ORBICULATE CARDINAL FISH SPHAERAMIA ORBICULARIS (CUVIER, 1828) (TELEOSTEI : APOGONIDAE) IN A KENYAN MANGROVE FOREST by Jan MEES Q, Godlove U. MWAMSOJO (2) & Enock O. WAKWABI (3) S u m m a r y . — The orbiculate cardinal fish Sphaeramia orbicularis is the most abundant teleost among the root system of the extensive mangrove forests bordering Gazi bay, Kenya. The species was never recorded from the bay proper and it can thus be considered to be a true mangrove resident. The sampled population clearly consisted of two cohorts : the modes were approxi mately 65 mm and 80 mm. Most individuals with standard lengths > 40 mm had mature gonads ; the number of eggs ranged from 4 700 to 10 000. S. orbicularis are carnivores, mainly feeding on small epi- and hyperbenthic crustaceans. Numerically, gammaridean amphipods and tanaids were the dominant prey categories in the stomachs of both size classes. Individuals belonging to the smaller cohort mainly supplemented their diet with harpac- ticoid copepods, while larger fishes also fed on postlarval brachyuran crabs and caridean shrimp. The latter two taxa were important prey items in gravimetrical terms. A preliminary analysis of the otoliths revealed 21 stress marks and 20 striations. An attempt to validate these growth rings indicated that the average age of fishes in the samples ranged from 11 (smaller cohort) to 15 (larger cohort) months. (1) Marine Biology Section, Zoology Institute, University of Gent, K.L. Ledeganckstraat, B-9000 Gent, Belgium. Email : [email protected] (2) Tanzania Fisheries Research Institute (TAFIRI), P.O. -

Heavy Metal Bioaccumulation in Commercial Lethrinidae Fish Species
Italian Journal of Food Safety 2017; volume 6:6607 Heavy metal bioaccumulation ration, industrials (paints, fertilisers, pesti- in commercial Lethrinidae fish cides, textile, leather and pharmaceuticals) Correspondence: Bhoyroo Vishwakalyan, and mine drainage (Ansari et al., 2004). Department of Food Sciences, Faculty of species in Mauritius Erosion, volcanism and magmatic activity Agriculture, University of Mauritius, Reduit, is said to cause atmospheric metal pollution Mauritius. Tel: +230 5797 8038. Bhanoo Saulick,1 responsible for dissolved metals such as Email: email: [email protected] Vishwakalyan Bhoyroo,1 arsenic (As), cadmium (Cd), copper (Cu), 2 Nadeem Nazurally, Iron (Fe), Nickel (Ni) and Zinc (Zn) to be Acknowledgements: we are thankful to Mrs Bhanooduth Lalljee1 present in the oceans (Chiarelli and M. Seetohul, Mrs M. Budhoo, Miss F. Roccheri, 2014). Rise in the level of heavy Jaumdally, Mrs K.P. Ghoorbin, Miss B. 1Department of Food Sciences, Faculty metals in the marine environment has Sohun, Mr S. Bundhun, Mr A. Soomaroo, Mr of Agriculture, University of Mauritius, A. Jhurrea, Mrs I.A Noormohamed, Mrs 2 caused severe problem to the marine organ- Reduit; Faculty of Ocean Sciences, ism and humans (Bashir et al., 2013). Due A.Sobhee, Mr P.S Baboololl and Mr L Junye for their support for laboratory analysis. We University of Mauritius, Reduit, to their capability to bio-accumulate heavy Mauritius are indebted to Mr V. Ramsahye and Mr metals, edible fishes have gained serious C.D.W Abdool from the Faculty of Science, concern and importance as the consumption Chemistry Department (UoM) for their help of wild and aqua-cultured fish increased and assistance with laboratory equipments. -

Changes in the Coral Reef Communities of Fagatele Bay National Marine Sanctuary and Tutuila Island (American Samoa), 1982-1995
Fagatele Bay National MarineSanctuary Science Series 2003-1 Changes in the coral reef communities of Fagatele Bay National Marine Sanctuary and Tutuila Island (American Samoa), 1982-1995 Charles Birkeland, University of Guam Richard H. Randall, University of Guam Alison L. Green, American Samoa Department of Marine and Wildlife Resources Barry D. Smith, University of Guam Suzanne Wilkins, University of Guam U.S. Department of Commerce American Samoa Government National Oceanic and Atmospheric Administration Department of Commerce National Ocean Service Environment Division Office of National Marine Sanctuaries Pago Pago, American Samoa May, 2003 DISCLAIMER Report content does not necessarily reflect the views and policies of the National Marine Sanctuary Program or the National oceanic and Atmospheric Administration, nor does the mention of trade names or commercial products constitute endorsement or recommendation for use. REPORT AVAILABILITY Electronic copies of this report may be downloaded from the National Marine Sanctuaries Program web site at www. Sanctuaries.nos.noaa.gov. Hard copies may be available from the following address: Fagatele Bay National Marine Sanctuary PO Box 4318 Pago Pago, As 96799 SUGGESTED CITATION Suggested citation: Birkeland, C.1, Randall, R.1, Green, A.2, Smith, B.1, and Wilkins, S. 1. 2003. Changes in the coral reef communities of Fagatele Bay NMS and Tutuila Island (American Samoa), 1982-1995. Fagatele Bay National Marine Sanctuary Science Series. Pago Pago, AS. p. 237 1 University of Guam Marine Laboratory, UOG Station, Mangilao, Guam 96923 2 Department of Marine and Wildlife Resources, Pago Pago, AS 96799 TABLE OF CONTENTS Abstract vii Coral Communities (by R.H. Randall and C. -
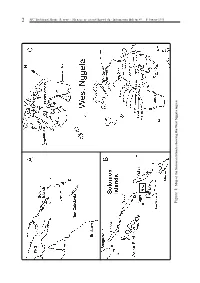
An Analysis of the West Nggela (Solomon Islands) Fish Taxonomy
2 SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 Map of the Solomon Islands showing West Nggela region Figure 1: Figure SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 3 What’s in a name? An analysis of the West Nggela (Solomon Islands) fish taxonomy. by Simon Foale 1 Introduction Lobotidae, Gerreidae, Sparidae, Ephippidae, Chaetodontidae, Pomacentridae, Cirhitidae, Accurate knowledge about the behaviour, biol- Polynemidae, Labridae, Opistognathidae, ogy and ecology of organisms comprising marine Trichonotidae, Pinguipedidae, Blenniidae, fisheries is a vital prerequisite for their manage- Gobiidae, Microdesmidae, Zanclidae, Bothidae, ment. Before beginning any study on local knowl- Pleuronectidae, and Soleidae. edge of marine fauna, a working knowledge of The English names of many species of fish vary their local names must be obtained. Moreover, a quite a bit, even within one country such as great deal of local knowledge can often emerge in Australia. For most of the species listed in the very process of obtaining names (Ruddle, Appendix 1, I have used the English names given 1994). A detailed treatment of the local naming by Randall et al. (1990). For species not included in system of West Nggela marine fauna is given in Randall et al. (1990), names from Kailola (1987a, b, this paper. 1991) were used. Methods Results Local names of fish were collected by asking Appendix 1 contains 350 unique Nggela folk people to provide the Nggela names for fishes taxa for cartilaginous and bony fishes, together from photographs in books featuring most of the with the scientific (Linnean) taxa they correspond common Indo-Pacific species (Randall et al., 1990 to and, where available, a brief note describing an and Myers, 1991). -

Lethrinus, Lethrinidae, Percoidei) from Three Areas Around Sulawesi (Indonesia) with Different Levels of Destructive Fishing
Volume 13, Supplementary Issue, December 2020 ISSN 1995-6673 JJBS Pages 637 - 646 Jordan Journal of Biological Sciences Landmark-based Morphometric and Meristic Variations in Emperors (Lethrinus, Lethrinidae, Percoidei) from Three Areas around Sulawesi (Indonesia) with Different Levels of Destructive Fishing Muhammad Afrisal1, Nurjirana1, Irmawati1, Yukio Iwatsuki2 and Andi Iqbal 3,* Burhanuddin 1Department of Fisheries Science, Faculty of Marine Science and Fisheries, Hasanuddin University, Indonesia; 2 Division of Fisheries Sciences, Faculty of Agriculture, Miyazaki University, Japan. 3 Marine Biology Laboratory, Faculty of Marine Science and Fisheries, Hasanuddin University, Makassar, Indonesia Received: December 30, 2019; Revised: February 23, 2020; Accepted: April 6, 2020 Abstract This study analysed the variation in morphometric and meristic characteristics among fishes in the genus Lethrinus from three areas around Sulawesi (Indonesia) with different levels of destructive fishing: Makassar, South Sulawesi, high; Manado, North Sulawesi, medium; and Wakatobi, Southeast Sulawesi, low. The research was conducted from June- November 2019. Morphometric characters (21) and meristic characters (8) of L. erythropterus, L. semicintus, L. obsoletus, L. ornatus, and L. harak were measured (30 specimens/species/site). Morphometric characters were compared between areas using one-way analysis of variance (ANOVA) with post-hoc Tukey Test. Characteristic traits and similarities between species/areas were evaluated using Multivariate Discriminant Function Analysis (DFA) test and tree diagram (dendrogram) analysis. For the five lethrinid species studied there were statistically significant differences (P<0.05) in morphometric characters between the Makassar population and the populations in Manado and Wakatobi. Interestingly, meristic count variability was greater in lethrinids from Makassar and Manado compared to those from the Wakatobi marine protected area.