Transcriptional Changes Involved in Atrophying Muscles During Prolonged Fasting in Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Program Nr: 1 from the 2004 ASHG Annual Meeting Mutations in A
Program Nr: 1 from the 2004 ASHG Annual Meeting Mutations in a novel member of the chromodomain gene family cause CHARGE syndrome. L.E.L.M. Vissers1, C.M.A. van Ravenswaaij1, R. Admiraal2, J.A. Hurst3, B.B.A. de Vries1, I.M. Janssen1, W.A. van der Vliet1, E.H.L.P.G. Huys1, P.J. de Jong4, B.C.J. Hamel1, E.F.P.M. Schoenmakers1, H.G. Brunner1, A. Geurts van Kessel1, J.A. Veltman1. 1) Dept Human Genetics, UMC Nijmegen, Nijmegen, Netherlands; 2) Dept Otorhinolaryngology, UMC Nijmegen, Nijmegen, Netherlands; 3) Dept Clinical Genetics, The Churchill Hospital, Oxford, United Kingdom; 4) Children's Hospital Oakland Research Institute, BACPAC Resources, Oakland, CA. CHARGE association denotes the non-random occurrence of ocular coloboma, heart defects, choanal atresia, retarded growth and development, genital hypoplasia, ear anomalies and deafness (OMIM #214800). Almost all patients with CHARGE association are sporadic and its cause was unknown. We and others hypothesized that CHARGE association is due to a genomic microdeletion or to a mutation in a gene affecting early embryonic development. In this study array- based comparative genomic hybridization (array CGH) was used to screen patients with CHARGE association for submicroscopic DNA copy number alterations. De novo overlapping microdeletions in 8q12 were identified in two patients on a genome-wide 1 Mb resolution BAC array. A 2.3 Mb region of deletion overlap was defined using a tiling resolution chromosome 8 microarray. Sequence analysis of genes residing within this critical region revealed mutations in the CHD7 gene in 10 of the 17 CHARGE patients without microdeletions, including 7 heterozygous stop-codon mutations. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Deubiquitinases in Cancer: New Functions and Therapeutic Options
Oncogene (2012) 31, 2373–2388 & 2012 Macmillan Publishers Limited All rights reserved 0950-9232/12 www.nature.com/onc REVIEW Deubiquitinases in cancer: new functions and therapeutic options JM Fraile1, V Quesada1, D Rodrı´guez, JMP Freije and C Lo´pez-Otı´n Departamento de Bioquı´mica y Biologı´a Molecular, Facultad de Medicina, Instituto Universitario de Oncologı´a, Universidad de Oviedo, Oviedo, Spain Deubiquitinases (DUBs) have fundamental roles in the Hunter, 2010). Consistent with the functional relevance ubiquitin system through their ability to specifically of proteases in these processes, alterations in their deconjugate ubiquitin from targeted proteins. The human structure or in the mechanisms controlling their genome encodes at least 98 DUBs, which can be grouped spatiotemporal expression patterns and activities cause into 6 families, reflecting the need for specificity in diverse pathologies such as arthritis, neurodegenerative their function. The activity of these enzymes affects the alterations, cardiovascular diseases and cancer. Accord- turnover rate, activation, recycling and localization ingly, many proteases are an important focus of of multiple proteins, which in turn is essential for attention for the pharmaceutical industry either as drug cell homeostasis, protein stability and a wide range of targets or as diagnostic and prognostic biomarkers signaling pathways. Consistent with this, altered DUB (Turk, 2006; Drag and Salvesen, 2010). function has been related to several diseases, including The recent availability of the genome sequence cancer. Thus, multiple DUBs have been classified as of different organisms has facilitated the identification oncogenes or tumor suppressors because of their regula- of their entire protease repertoire, which has been tory functions on the activity of other proteins involved in defined as degradome (Lopez-Otin and Overall, 2002). -

1 Supporting Information for a Microrna Network Regulates
Supporting Information for A microRNA Network Regulates Expression and Biosynthesis of CFTR and CFTR-ΔF508 Shyam Ramachandrana,b, Philip H. Karpc, Peng Jiangc, Lynda S. Ostedgaardc, Amy E. Walza, John T. Fishere, Shaf Keshavjeeh, Kim A. Lennoxi, Ashley M. Jacobii, Scott D. Rosei, Mark A. Behlkei, Michael J. Welshb,c,d,g, Yi Xingb,c,f, Paul B. McCray Jr.a,b,c Author Affiliations: Department of Pediatricsa, Interdisciplinary Program in Geneticsb, Departments of Internal Medicinec, Molecular Physiology and Biophysicsd, Anatomy and Cell Biologye, Biomedical Engineeringf, Howard Hughes Medical Instituteg, Carver College of Medicine, University of Iowa, Iowa City, IA-52242 Division of Thoracic Surgeryh, Toronto General Hospital, University Health Network, University of Toronto, Toronto, Canada-M5G 2C4 Integrated DNA Technologiesi, Coralville, IA-52241 To whom correspondence should be addressed: Email: [email protected] (M.J.W.); yi- [email protected] (Y.X.); Email: [email protected] (P.B.M.) This PDF file includes: Materials and Methods References Fig. S1. miR-138 regulates SIN3A in a dose-dependent and site-specific manner. Fig. S2. miR-138 regulates endogenous SIN3A protein expression. Fig. S3. miR-138 regulates endogenous CFTR protein expression in Calu-3 cells. Fig. S4. miR-138 regulates endogenous CFTR protein expression in primary human airway epithelia. Fig. S5. miR-138 regulates CFTR expression in HeLa cells. Fig. S6. miR-138 regulates CFTR expression in HEK293T cells. Fig. S7. HeLa cells exhibit CFTR channel activity. Fig. S8. miR-138 improves CFTR processing. Fig. S9. miR-138 improves CFTR-ΔF508 processing. Fig. S10. SIN3A inhibition yields partial rescue of Cl- transport in CF epithelia. -

Beyond K48 and K63: Non-Canonical Protein Ubiquitination
Tracz and Bialek Cell Mol Biol Lett (2021) 26:1 https://doi.org/10.1186/s11658‑020‑00245‑6 Cellular & Molecular Biology Letters REVIEW LETTER Open Access Beyond K48 and K63: non‑canonical protein ubiquitination Michal Tracz and Wojciech Bialek* *Correspondence: [email protected] Abstract Faculty of Biotechnology, Protein ubiquitination has become one of the most extensively studied post-trans- University of Wroclaw, Wroclaw, Poland lational modifcations. Originally discovered as a critical element in highly regulated proteolysis, ubiquitination is now regarded as essential for many other cellular pro- cesses. This results from the unique features of ubiquitin (Ub) and its ability to form various homo- and heterotypic linkage types involving one of the seven diferent lysine residues or the free amino group located at its N-terminus. While K48- and K63-linked chains are broadly covered in the literature, the other types of chains assembled through K6, K11, K27, K29, and K33 residues deserve equal attention in the light of the latest discoveries. Here, we provide a concise summary of recent advances in the feld of these poorly understood Ub linkages and their possible roles in vivo. Keywords: Ubiquitin, Non-canonical, Atypical ubiquitination, Ubiquitin chains Introduction Protein ubiquitination (interchangeably called ubiquitylation) involves the covalent attachment of the 76-amino acid eukaryotic molecule ubiquitin (Ub) to substrate pro- teins. An enzymatic cascade of a Ub activating enzyme (E1), Ub-conjugating enzymes (E2s), and Ub ligases (E3s) governs the process, which is now recognized as an essential post-translational protein modifcation (PTM) of eukaryotic cells [1]. Ubiquitination is initiated by E1, an enzyme requiring ATP for the activation of Ub, which forms a thi- oester bond between the C-terminal Gly carboxyl group of Ub and its active site Cys. -

Characterization of the Small RNA Transcriptomes of Cell Protrusions and Cell Bodies of Highly Metastatic Hepatocellular Carcinoma Cells Via RNA Sequencing
ONCOLOGY LETTERS 22: 568, 2021 Characterization of the small RNA transcriptomes of cell protrusions and cell bodies of highly metastatic hepatocellular carcinoma cells via RNA sequencing WENPIN CAI1*, JINGZHANG JI2*, BITING WU2*, KAIXUAN HAO2, PING REN2, YU JIN2, LIHONG YANG2, QINGCHAO TONG2 and ZHIFA SHEN2 1Department of Laboratory Medicine, Wen Zhou Traditional Chinese Medicine Hospital; 2Zhejiang Provincial Key Laboratory of Medical Genetics, Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, Zhejiang 325035, P.R. China Received July 11, 2020; Accepted February 23, 2021 DOI: 10.3892/ol.2021.12829 Abstract. Increasing evidence suggest that hepatocellular differentially expressed miRNAs and circRNAs. The interac‑ carcinoma (HCC) HCCLM3 cells initially develop pseudo‑ tion maps between miRNAs and circRNAs were constructed, podia when they metastasize, and microRNAs (miRNAs/miRs) and signaling pathway maps were analyzed to determine the and circular RNAs (circRNAs) have been demonstrated to molecular mechanism and regulation of the differentially serve important roles in the development, progression and expressed miRNAs and circRNAs. Taken together, the results metastasis of cancer. The present study aimed to isolate the of the present study suggest that the Boyden chamber assay cell bodies (CBs) and cell protrusions (CPs) from HCCLM3 can be used to effectively isolate the somatic CBs and CPs of cells, and screen the miRNAs and circRNAs associated with HCC, which can be used to screen the miRNAs and circRNAs HCC infiltration and metastasis in CBs and CPs. The Boyden associated with invasion and metastasis of HCC. chamber assay has been confirmed to effectively isolate the CBs and CPs from HCCLM3 cells via observation of microtu‑ Introduction bule immunofluorescence, DAPI staining and nuclear protein H3 western blotting. -

Post-Translational Modification of MRE11: Its Implication in DDR And
G C A T T A C G G C A T genes Review Post-Translational Modification of MRE11: Its Implication in DDR and Diseases Ruiqing Lu 1,† , Han Zhang 2,† , Yi-Nan Jiang 1, Zhao-Qi Wang 3,4, Litao Sun 5,* and Zhong-Wei Zhou 1,* 1 School of Medicine, Sun Yat-Sen University, Shenzhen 518107, China; [email protected] (R.L.); [email protected] (Y.-N.J.) 2 Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College; Kunming 650118, China; [email protected] 3 Leibniz Institute on Aging–Fritz Lipmann Institute (FLI), 07745 Jena, Germany; zhao-qi.wang@leibniz-fli.de 4 Faculty of Biological Sciences, Friedrich-Schiller-University of Jena, 07745 Jena, Germany 5 School of Public Health (Shenzhen), Sun Yat-Sen University, Shenzhen 518107, China * Correspondence: [email protected] (L.S.); [email protected] (Z.-W.Z.) † These authors contributed equally to this work. Abstract: Maintaining genomic stability is vital for cells as well as individual organisms. The meiotic recombination-related gene MRE11 (meiotic recombination 11) is essential for preserving genomic stability through its important roles in the resection of broken DNA ends, DNA damage response (DDR), DNA double-strand breaks (DSBs) repair, and telomere maintenance. The post-translational modifications (PTMs), such as phosphorylation, ubiquitination, and methylation, regulate directly the function of MRE11 and endow MRE11 with capabilities to respond to cellular processes in promptly, precisely, and with more diversified manners. Here in this paper, we focus primarily on the PTMs of MRE11 and their roles in DNA response and repair, maintenance of genomic stability, as well as their Citation: Lu, R.; Zhang, H.; Jiang, association with diseases such as cancer. -

Relevance of Translation Initiation in Diffuse Glioma Biology and Its
cells Review Relevance of Translation Initiation in Diffuse Glioma Biology and its Therapeutic Potential Digregorio Marina 1, Lombard Arnaud 1,2, Lumapat Paul Noel 1, Scholtes Felix 1,2, Rogister Bernard 1,3 and Coppieters Natacha 1,* 1 Laboratory of Nervous System Disorders and Therapy, GIGA-Neurosciences Research Centre, University of Liège, 4000 Liège, Belgium; [email protected] (D.M.); [email protected] (L.A.); [email protected] (L.P.N.); [email protected] (S.F.); [email protected] (R.B.) 2 Department of Neurosurgery, CHU of Liège, 4000 Liège, Belgium 3 Department of Neurology, CHU of Liège, 4000 Liège, Belgium * Correspondence: [email protected] Received: 18 October 2019; Accepted: 26 November 2019; Published: 29 November 2019 Abstract: Cancer cells are continually exposed to environmental stressors forcing them to adapt their protein production to survive. The translational machinery can be recruited by malignant cells to synthesize proteins required to promote their survival, even in times of high physiological and pathological stress. This phenomenon has been described in several cancers including in gliomas. Abnormal regulation of translation has encouraged the development of new therapeutics targeting the protein synthesis pathway. This approach could be meaningful for glioma given the fact that the median survival following diagnosis of the highest grade of glioma remains short despite current therapy. The identification of new targets for the development of novel therapeutics is therefore needed in order to improve this devastating overall survival rate. This review discusses current literature on translation in gliomas with a focus on the initiation step covering both the cap-dependent and cap-independent modes of initiation. -

Product Data Sheet
For research purposes only, not for human use Product Data Sheet Anti-UBE4A Antibody Catalog # Source Reactivity Applications CPA2384 Rabbit H, M, R WB Description Rabbit polyclonal antibody to UBE4A Immunogen KLH-conjugated synthetic peptide encompassing a sequence within the N-term region of human UBE4A. The exact sequence is proprietary. Purification The antibody was purified by immunogen affinity chromatography. Specificity Recognizes endogenous levels of UBE4A protein. Clonality Polyclonal Conjugation Form Liquid in 0.42% Potassium phosphate, 0.87% Sodium chloride, pH 7.3, 30% glycerol, and 0.01% sodium azide. Dilution WB (1/500 - 1/1000) Gene Symbol UBE4A Alternative Names KIAA0126; Ubiquitin conjugation factor E4 A Entrez Gene 9354 (Human); 315608 (Rat) SwissProt Q14139 (Human); Q6P7A2 (Rat) Storage/Stability Shipped at 4°C. Upon delivery aliquot and store at -20°C for one year. Avoid freeze/thaw cycles. Application key: E- ELISA, WB- Western blot, IH- Immunohistochemistry, IF- Immunofluorescence, FC- Flow cytometry, IC- Immunocytochemistry, IP- Immunoprecipitation, ChIP- Chromatin Immunoprecipitation, EMSA- Electrophoretic Mobility Shift Assay, BL- Blocking, SE- Sandwich ELISA, CBE- Cell-based ELISA, RNAi- RNA interference Species reactivity key: H- Human, M- Mouse, R- Rat, B- Bovine, C- Chicken, D- Dog, G- Goat, Mk- Monkey, P- Pig, Rb- Rabbit, S- Sheep, Z- Zebrafish COHESION BIOSCIENCES LIMITED WEB ORDER SUPPORT CUSTOM www.cohesionbio.com [email protected] [email protected] [email protected] For research purposes -

NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-7-2018 Decipher Mechanisms by which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer Jairo Ramos [email protected] Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Clinical Epidemiology Commons Recommended Citation Ramos, Jairo, "Decipher Mechanisms by which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer" (2018). FIU Electronic Theses and Dissertations. 3872. https://digitalcommons.fiu.edu/etd/3872 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida DECIPHER MECHANISMS BY WHICH NUCLEAR RESPIRATORY FACTOR ONE (NRF1) COORDINATES CHANGES IN THE TRANSCRIPTIONAL AND CHROMATIN LANDSCAPE AFFECTING DEVELOPMENT AND PROGRESSION OF INVASIVE BREAST CANCER A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in PUBLIC HEALTH by Jairo Ramos 2018 To: Dean Tomás R. Guilarte Robert Stempel College of Public Health and Social Work This dissertation, Written by Jairo Ramos, and entitled Decipher Mechanisms by Which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer, having been approved in respect to style and intellectual content, is referred to you for judgment. -
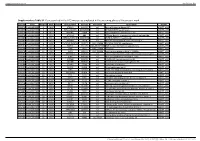
Supplementary Table S1. Genes Printed in the HC5 Microarray Employed in the Screening Phase of the Present Work
Supplementary material Ann Rheum Dis Supplementary Table S1. Genes printed in the HC5 microarray employed in the screening phase of the present work. CloneID Plate Position Well Length GeneSymbol GeneID Accession Description Vector 692672 HsxXG013989 2 B01 STK32A 202374 null serine/threonine kinase 32A pANT7_cGST 692675 HsxXG013989 3 C01 RPS10-NUDT3 100529239 null RPS10-NUDT3 readthrough pANT7_cGST 692678 HsxXG013989 4 D01 SPATA6L 55064 null spermatogenesis associated 6-like pANT7_cGST 692679 HsxXG013989 5 E01 ATP1A4 480 null ATPase, Na+/K+ transporting, alpha 4 polypeptide pANT7_cGST 692689 HsxXG013989 6 F01 ZNF816-ZNF321P 100529240 null ZNF816-ZNF321P readthrough pANT7_cGST 692691 HsxXG013989 7 G01 NKAIN1 79570 null Na+/K+ transporting ATPase interacting 1 pANT7_cGST 693155 HsxXG013989 8 H01 TNFSF12-TNFSF13 407977 NM_172089 TNFSF12-TNFSF13 readthrough pANT7_cGST 693161 HsxXG013989 9 A02 RAB12 201475 NM_001025300 RAB12, member RAS oncogene family pANT7_cGST 693169 HsxXG013989 10 B02 SYN1 6853 NM_133499 synapsin I pANT7_cGST 693176 HsxXG013989 11 C02 GJD3 125111 NM_152219 gap junction protein, delta 3, 31.9kDa pANT7_cGST 693181 HsxXG013989 12 D02 CHCHD10 400916 null coiled-coil-helix-coiled-coil-helix domain containing 10 pANT7_cGST 693184 HsxXG013989 13 E02 IDNK 414328 null idnK, gluconokinase homolog (E. coli) pANT7_cGST 693187 HsxXG013989 14 F02 LYPD6B 130576 null LY6/PLAUR domain containing 6B pANT7_cGST 693189 HsxXG013989 15 G02 C8orf86 389649 null chromosome 8 open reading frame 86 pANT7_cGST 693194 HsxXG013989 16 H02 CENPQ 55166 -

Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name ubiquitin conjugation factor E4 A Gene Symbol Ube4a Organism Rat Gene Summary Description Not Available Gene Aliases Not Available RefSeq Accession No. NM_207610 UniGene ID Rn.102204 Ensembl Gene ID ENSRNOG00000026833 Entrez Gene ID 315608 Assay Information Unique Assay ID qRnoCIP0022949 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENSRNOT00000021321 Amplicon Context Sequence GCGATCAGCAGAGAGGTTTAAAGTTGCAGAGGAAGTCATGCTGATCAAGTGGTT TCCTGGATCTTGAAGCAGTAACCGAGCAAAGATGGCCTGCTCCACGTTGCTCAT ATCCAGCCAGTCTTG Amplicon Length (bp) 93 Chromosome Location 8:47874133-47875261 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 94 R2 0.9997 cDNA Cq 21.53 cDNA Tm (Celsius) 83 gDNA Cq 42.59 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Ube4a, Rat Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect.