Atlantic Cod (Gadus Morhua) in the Southern Gulf of St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

Atlantic Cod (Gadus Morhua) Off Newfoundland and Labrador Determined from Genetic Variation
COSEWIC Assessment and Update Status Report on the Atlantic Cod Gadus morhua Newfoundland and Labrador population Laurentian North population Maritimes population Arctic population in Canada Newfoundland and Labrador population - Endangered Laurentian North population - Threatened Maritimes population - Special Concern Arctic population - Special Concern 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and update status report on the Atlantic cod Gadus morhua in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 76 pp. Production note: COSEWIC would like to acknowledge Jeffrey A. Hutchings for writing the update status report on the Atlantic cod Gadus morhua, prepared under contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Rapport du COSEPAC sur la situation de la morue franche (Gadus morhua) au Canada Cover illustration: Atlantic Cod — Line drawing of Atlantic cod Gadus morhua by H.L. Todd. Image reproduced with permission from the Smithsonian Institution, NMNH, Division of Fishes. Her Majesty the Queen in Right of Canada, 2003 Catalogue No.CW69-14/311-2003-IN ISBN 0-662-34309-3 Recycled paper COSEWIC Assessment Summary Assessment summary — May 2003 Common name Atlantic cod (Newfoundland and Labrador population) Scientific name Gadus morhua Status Endangered Reason for designation Cod in the inshore and offshore waters of Labrador and northeastern Newfoundland, including Grand Bank, having declined 97% since the early 1970s and more than 99% since the early 1960s, are now at historically low levels. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

A Review of Spatial and Temporal Variation in Grey and Common Seal Diet in the United Kingdom and Ireland Susie L
Journal of the Marine Biological Association of the United Kingdom, 2012, 92(8), 1711–1722. # Marine Biological Association of the United Kingdom, 2012 doi:10.1017/S0025315411002050 A review of spatial and temporal variation in grey and common seal diet in the United Kingdom and Ireland susie l. brown1,5, stuart bearhop2, chris harrod1,3 and robbie a. mcdonald4 1School of Biological Sciences, Queen’s University Belfast, Medical Biology Centre, 97 Lisburn Road, Belfast, BT9 7BL, 2Centre for Ecology and Conservation, School of Biosciences, University of Exeter, Cornwall Campus, Penryn, Cornwall, TR10 9EZ, 3Facultad de Recursos del Mar, Instituto de Investigaciones Oceanolo´gicas, Universidad Antofagasta, Avenida Angamos 601, Antofagasta, Chile, 4Environment and Sustainability Institute, University of Exeter, Cornwall Campus, Penryn, Cornwall TR10 9EZ, 5Current address: Department of Zoology, Ecology and Plant Science, Cork Enterprise Centre, University College Cork, Distillery Fields, North Mall, Cork Knowledge about the diet of fish-eating predators is critical when evaluating conflicts with the fishing industry. Numerous primary studies have examined the diet of grey seals Halichoerus grypus and common seals Phoca vitulina in a bid to under- stand the ecology of these predators. However, studies of large-scale spatial and temporal variation in seal diet are limited. Therefore this review combines the results of seal diet studies published between 1980 and 2000 to examine how seal diet varies at a range of spatial and temporal scales. Our results revealed extensive spatial variation in gadiform, perciform and flatfish consumption, likely reflecting variation in prey availability. Flatfish and gadiform consumption varied between years, reflect- ing changes in fish assemblages as a consequence of factors such as varying fishing pressures, climate change and natural fluc- tuations in populations. -
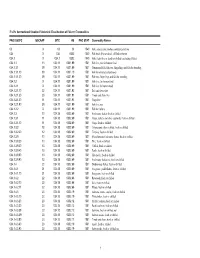
FAO's International Standard Statistical Classification of Fishery Commodities
FAO's International Standard Statistical Classification of Fishery Commodities FAO ISSCFC ISSCAAP SITC HS FAO STAT Commodity Names 03 X 03 03 1540 Fish, crustaceans, molluscs and preparations 034 X 034 0302 1540 Fish fresh (live or dead), chilled or frozen 034.1 X 034.1 0302 1540 Fish, fresh (live or dead) or chilled (excluding fillets) 034.1.1 13 034.11 0301.99 1501 Fish live, not for human food 034.1.1.1 39 034.11 0301.99 1501 Ornamental fish, fish ova, fingerlings and fish for breeding 034.1.1.1.10 39 034.11 0301.10 1501 Fish for ornamental purposes 034.1.1.1.20 39 034.11 0301.99 1501 Fish ova, fingerlings and fish for breeding 034.1.2 X 034.110301.99 1501 Fish live, for human food 034.1.2.1 X 034.110301.99 1501 Fish live for human food 034.1.2.1.10 22 034.11 0301.92 1501 Eels and elvers live 034.1.2.1.20 23 034.11 0301.91 1501 Trouts and chars live 034.1.2.1.30 11 034.11 0301.93 1501 Carps live 034.1.2.1.90 39 034.11 0301.99 1501 Fish live, nei 034.1.2.2 X 034.110301.99 1501 Fish for culture 034.1.3 10 034.18 0302.69 1501 Freshwater fishes, fresh or chilled 034.1.3.1 11 034.18 0302.69 1501 Carps, barbels and other cyprinids, fresh or chilled 034.1.3.1.10 11 034.18 0302.69 1501 Carps, fresh or chilled 034.1.3.2 12 034.18 0302.69 1501 Tilapias and other cichlids, fresh or chilled 034.1.3.2.20 12 034.18 0302.69 1501 Tilapias, fresh or chilled 034.1.3.9 10 034.18 0302.69 1501 Miscellaneous freshwater fishes, fresh or chilled 034.1.3.9.20 13 034.18 0302.69 1501 Pike, fresh or chilled 034.1.3.9.30 13 034.18 0302.69 1501 Catfish, fresh or -
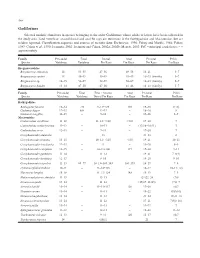
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Genetic Structure in the Amazonian Catfish Brachyplatystoma Rousseauxii
Genetica (2014) 142:323–336 DOI 10.1007/s10709-014-9777-2 Genetic structure in the Amazonian catfish Brachyplatystoma rousseauxii: influence of life history strategies F. M. Carvajal-Vallejos • F. Duponchelle • E. Desmarais • F. Cerqueira • S. Querouil • J. Nun˜ez • C. Garcı´a • J.-F. Renno Received: 21 December 2013 / Accepted: 7 July 2014 / Published online: 20 July 2014 Ó Springer International Publishing Switzerland 2014 Abstract The Dorado or Plateado (Gilded catfish) Bayesian analyses revealed at least three clusters in Brachyplatystoma rousseauxii (Pimelodidae, Siluriformes) admixture in the five locations sampled in the Bolivian is a commercially valuable migratory catfish performing Amazon, whereas only two of these clusters were observed the largest migration in freshwaters: from the Amazonian in the Western Amazon. Considering the migratory headwaters in the Andean foothills (breeding area) to the behaviour of B. rousseauxii, different life history strategies, Amazon estuary (nursery area). In spite of its importance to including homing, are proposed to explain the cluster dis- inform management and conservation efforts, the genetic tribution. Our results are discussed in the light of the variability of this species has only recently begun to be numerous threats to the species survival in the Madera studied. The aim of the present work was to determine the basin, in particular dam and reservoir construction. population genetic structure of B. rousseauxii in two regions: the Upper Madera Basin (five locations in the Keywords Bolivia Á Madera River Á Population Bolivian Amazon) and the Western Amazon Basin (one structure Á Microsatellites Á Siluriformes Á Pimelodidae regional sample from the Uyucalı´–Napo–Maran˜on–Ama- zon basin, Peru). -

Zootaxa 3165: 1–24 (2012) ISSN 1175-5326 (Print Edition) Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (Online Edition)
Zootaxa 3165: 1–24 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) A new species of grenadier, genus Macrourus (Teleostei, Gadiformes, Macrouridae) from the southern hemisphere and a revision of the genus PETER MCMILLAN1, TOMIO IWAMOTO2, ANDREW STEWART3 & PETER J SMITH4 1National Institute of Water and Atmospheric Research Ltd, Private Bag 14901, Wellington, 6241, New Zealand. E-mail: [email protected] 2Department of Ichthyology, California Academy of Sciences, 55 Concourse Drive, San Francisco, CA 94118, USA. E-mail: [email protected] 3Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington, New Zealand 4Museum Victoria, GPO Box 666, Melbourne, Victoria 3001, Australia Abstract A new Macrourus species from the southern hemisphere is described. It was first recognised from the Ross Sea, Antarctica after specimens sampled during the International Polar Year in 2008 showed significant genetic differences (C01) among those initially identified as M. whitsoni (Regan). M. caml sp. nov. has 8 (rarely 7 or 9) pelvic fin rays, a band (2–3 rows) of small uniform-sized teeth in the lower jaw, lacks an outer row of enlarged teeth in the upper jaw, 30–40 scales in a di- agonal row from anal fin origin to lateral line, ventral surface of the head is mostly scaled, except for scaleless areas an- terior to the mouth and on the anterior half of the lower jaw. M caml sp. nov. is large, reaching at least 890 mm TL and appears to be abundant. Numerous specimens caught by commercial bottom longline vessels fishing in the Ross Sea are held at Museum of New Zealand Te Papa Tongarewa, Wellington New Zealand. -

Mediterranean Sea
OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi MEDITERRANEAN The IUCN Red List of Threatened Species™ – Regional Assessment OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi The IUCN Red List of Threatened Species™ – Regional Assessment Compilers: Dania Abdul Malak Mediterranean Species Programme, IUCN Centre for Mediterranean Cooperation, calle Marie Curie 22, 29590 Campanillas (Parque Tecnológico de Andalucía), Málaga, Spain Suzanne R. Livingstone Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, c/o Conservation International, Arlington, VA 22202, USA David Pollard Applied Marine Conservation Ecology, 7/86 Darling Street, Balmain East, New South Wales 2041, Australia; Research Associate, Department of Ichthyology, Australian Museum, Sydney, Australia Beth A. Polidoro Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Annabelle Cuttelod Red List Unit, IUCN Species Programme, 219c Huntingdon Road, Cambridge CB3 0DL,UK Michel Bariche Biology Departement, American University of Beirut, Beirut, Lebanon Murat Bilecenoglu Department of Biology, Faculty of Arts and Sciences, Adnan Menderes University, 09010 Aydin, Turkey Kent E. Carpenter Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Bruce B. -

Checklist of Kansas Fishes
CHECKLIST OF KANSAS FISHES From "A Checklist of the Vertebrate Animals of Kansas", second edition, 1999, by George Potts, Joseph Collins and Kate Shaw (Species marked with an asterisk * are extirpated from the wild in Kansas.) 142 Species REFERENCE: Fishes in Kansas, 2nd edition, 1995 By Frank Cross and Joseph Collins, KU Press Order of Lampreys (Petromyzontiformes) Family Petromyzontidae Chestnut Lamprey - Ichthyomyzon castaneus Order of Sturgeons and Paddlefish (Acipenseriformes) Family Acipenseridae Lake Sturgeon - Acipenser fulvescens Pallid Sturgeon - Scaphirhynchus albus Shovelnose Sturgeon - Scaphirhynchus platorynchus Family Polyodontidae Paddlefish - Polyodon spathula Order of Gars (Semionotiformes) Family Lepisosteidae Spotted Gar - Lepisosteus oculatus Longnose Gar - Lepisosteus osseus Shortnose Gar - Lepisosteus platostomus Order of Bowfins (Amiiformes) Family Amiidae Bowfin - Amia calva Order of Bony-tongued fishes (Osteoglossiformes) Family Hiodontidae Goldeye - Hiodon alosoides * Mooneye - Hiodon tergisus Order of Eels (Anguilliformes) Family Anguillidae American Eel - Anguilla rostrata Order of Herrings (Clupeiformes) Family Clupeidae Skipjack Herring - Alosa chrysochloris Gizzard Shad - Dorosoma cepedianum Threadfin Shad - Dorosoma petenense Page 1 of 5 Order of Carp-like fishes (Cypriniformes) Family Cyprinidae Central Stoneroller - Campostoma anomalum Goldfish - Carassius auratus Grass Carp - Ctenopharyngodon idella Bluntface Shiner - Cyprinella camura Red Shiner - Cyprinella lutrensis Spotfin Shiner - Cyprinella spiloptera