Biodiversity Assessment of the Eastern Caribbean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environment Outlook
CARIBBEAN ENVIRONMENT OUTLOOK Special Edition for the Mauritius International Meeting for the 10-year Review of the Barbados Programme of Action for the Sustainable Development of Small Island Developing States CARICOM CARIBBEAN — ENVIRONMENT OUTLOOK ACKNOWLEDGEMENTS Editor Cox (Bahamas), Leonie Barnaby (Jamaica), Patrick Sherry Heileman, Consultant McConney (Barbados), Enrique Dalmau (Cuba), Patricia Aquing (St Lucia), Reynold Murray (St Vincent), Navin Lead authors Chandarpal (Guyana), Conrod Hunte (Antigua and Leslie John Walling (Belize), Dr Charles Douglas Barbuda), Milton Haughton (Belize), Bishnu Persaud (Jamaica), Maurice Mason (Jamaica) and Marcia (Guyana), Chris Corbin (St Lucia), Kelvin Penn (British Chevannes-Creary (Jamaica) Virgin Islands), Edwin Carrington (Guyana), Jose L. Gerhartz (Jamaica), Sharon Lindo (Belize), Leonardo The United Nations Environment Programme (UNEP) Nurse (Barbados), Byron Blake (Guyana), Ricardo would like to thank the following individuals and Sanchez Sosa (Mexico) and Mark Griffith (Mexico). institutions who played a vital role in the Thanks go to Diane Quarless (SIDS Unit) and United production of this report: Nations Department for Economic and Social Affairs (UNDESA) for facilitating this consultation. The Caribbean Community Secretariat and the University of the West Indies Centre for Environment Sincere appreciation is also extended to Arthur Dahl and Development (UWICED) for being the partners in (Consultant Advisor, UNEP), Espen Ronneberg (UNDESA) this project.The Caribbean Community Climate -

Eastern Africa's Manufacturing Sector: a National Validation Workshop on November 12Th 2013
Eastern Africa’s Manufacturing Sector Promoting Technology, Innovation, Productivity And Linkages SEYCHELLES COUNTRY REPORT October 2014 EASTERN AFRICA’S MANUFACTURING SECTOR Promoting technology, innovation, productivity and linkages SEYCHELLES COUNTRY REPORT October 2014 EASTERN AFRICA’S MANUFACTURING SECTOR - SEYCHELLES COUNTRY REPORT THE AFRICAN DEVELOPMENT BANK GROUP The production of this report has been coordinated by the African Development Bank (AfBD). Designations employed in this publication do not imply the expression of any opinion of the institution concerning the legal status of any country, or the limitation of its frontier. While efforts have been made to present reliable information, the AfDB accepts no responsibility whatsoever for any consequences of its use. Vice President: Zondo Sakala Regional Director (EARC): Gabriel Negatu Lead Economists: Stefan Muller, Abraham Mwenda Chief Regional Economist and Task Manager of the Report: Tilahun Temesgen Senior Country Economist: Susan Mpande Copyright 2014 – AFRICAN DEVELOPMENT BANK GROUP Photo Credits: AfDB photo files. PUBLISHED BY African Development Bank Group – Eastern Africa Regional Resource Centre (EARC) Khushee Tower Longonot Road, Upper Hill Nairobi, Kenya Phone: (254) 20 2712925/26/28 Fax: (254) 202712938 Email: [email protected] Website: www.afdb.org TABLE OF CONTENTS Executive Summary..................................................................................................................................................................ix Introduction..............................................................................................................................................................................xii -

Mapping the Information Environment in the Pacific Island Countries: Disruptors, Deficits, and Decisions
December 2019 Mapping the Information Environment in the Pacific Island Countries: Disruptors, Deficits, and Decisions Lauren Dickey, Erica Downs, Andrew Taffer, and Heidi Holz with Drew Thompson, S. Bilal Hyder, Ryan Loomis, and Anthony Miller Maps and graphics created by Sue N. Mercer, Sharay Bennett, and Michele Deisbeck Approved for Public Release: distribution unlimited. IRM-2019-U-019755-Final Abstract This report provides a general map of the information environment of the Pacific Island Countries (PICs). The focus of the report is on the information environment—that is, the aggregate of individuals, organizations, and systems that shape public opinion through the dissemination of news and information—in the PICs. In this report, we provide a current understanding of how these countries and their respective populaces consume information. We map the general characteristics of the information environment in the region, highlighting trends that make the dissemination and consumption of information in the PICs particularly dynamic. We identify three factors that contribute to the dynamism of the regional information environment: disruptors, deficits, and domestic decisions. Collectively, these factors also create new opportunities for foreign actors to influence or shape the domestic information space in the PICs. This report concludes with recommendations for traditional partners and the PICs to support the positive evolution of the information environment. This document contains the best opinion of CNA at the time of issue. It does not necessarily represent the opinion of the sponsor or client. Distribution Approved for public release: distribution unlimited. 12/10/2019 Cooperative Agreement/Grant Award Number: SGECPD18CA0027. This project has been supported by funding from the U.S. -

LEGISLATIVE RESOLUTION Commemorating the 32Nd Anniversary of the Inde- Pendence of Antigua and Barbuda
LEGISLATIVE RESOLUTION commemorating the 32nd Anniversary of the Inde- pendence of Antigua and Barbuda WHEREAS, It is the sense of this Legislative Body to recognize and pay just tribute to the cultural heritage of the ethnic groups which comprise and contribute to the richness and diversity of the community of the State of New York; and WHEREAS, Attendant to such concern, and in keeping with its time-ho- nored traditions, it is the intent of this Legislative Body to commem- orate the 32nd Anniversary of the Independence of Antigua and Barbuda; and WHEREAS, The people of this great State and nation and Antigua and Barbuda enjoy a deep and abiding relationship rooted in kinship and culture; and WHEREAS, Antigua and Barbuda's Independence Day is celebrated on November 1st of every year; and WHEREAS, Antigua and Barbuda is a small island country in the Northern Leeward Islands of the Caribbean Sea; the country consists of three islands, Antigua, Barbuda and Redonda, with an estimated population of around 80,000 inhabitants; and WHEREAS, Antigua and Barbuda's main economy is tourism; natives are known for their strong skills of arts and crafts; and WHEREAS, The majority of the population are descendants of African slaves who were brought to grow sugar cane and tobacco on the island in the colonial era - such as the British in the 17th century; and WHEREAS, In 1493, it is said the island of Antigua was sighted by Christopher Columbus and named after a Spanish church in Seville; it was not until 1632 when the British colonized Antigua, Barbuda -

The Gordian Knot: American and British Policy Concerning the Cyprus Issue: 1952-1974
THE GORDIAN KNOT: AMERICAN AND BRITISH POLICY CONCERNING THE CYPRUS ISSUE: 1952-1974 Michael M. Carver A Thesis Submitted to the Graduate College of Bowling Green State University in partial fulfillment of The requirements for the degree of MASTER OF ARTS May 2006 Committee: Dr. Douglas J. Forsyth, Advisor Dr. Gary R. Hess ii ABSTRACT Douglas J. Forsyth, Advisor This study examines the role of both the United States and Great Britain during a series of crises that plagued Cyprus from the mid 1950s until the 1974 invasion by Turkey that led to the takeover of approximately one-third of the island and its partition. Initially an ancient Greek colony, Cyprus was conquered by the Ottoman Empire in the late 16th century, which allowed the native peoples to take part in the island’s governance. But the idea of Cyprus’ reunification with the Greek mainland, known as enosis, remained a significant tenet to most Greek-Cypriots. The movement to make enosis a reality gained strength following the island’s occupation in 1878 by Great Britain. Cyprus was integrated into the British imperialist agenda until the end of the Second World War when American and Soviet hegemony supplanted European colonialism. Beginning in 1955, Cyprus became a battleground between British officials and terrorists of the pro-enosis EOKA group until 1959 when the independence of Cyprus was negotiated between Britain and the governments of Greece and Turkey. The United States remained largely absent during this period, but during the 1960s and 1970s came to play an increasingly assertive role whenever intercommunal fighting between the Greek and Turkish-Cypriot populations threatened to spill over into Greece and Turkey, and endanger the southeastern flank of NATO. -

Rising Islands
THESIS FROM THE DEPARTMENT OF HUMAN GEOGRAPHY JUNE 2015 Rising Islands Enhancing adaptive capacities in Kiribati through Migration with Dignity SANDRA DUONG Master's Thesis in Geography, 30 credits Supervisor: Martina Angela Caretta Department of Human Geography, Stockholm University www.humangeo.su.se ABSTRACT Duong, Sandra (2015). Rising Islands - Enhancing adaptive capacities in Kiribati through Migration with Dignity. Human Geography, advanced level, Master thesis for Master Exam in Human Geography, 30 ECTS credits. Supervisor: Martina Angela Caretta Language: English The main body of research within climate-change induced migration has focused on displacement migration. The “sinking islands” reference is often used to describe island states being in the forefront of climate change impacts, and their inhabitants at risk of becoming the first climate change refugees in history. The aim of this thesis is to understand what circumstances are needed for Kiribati’s ‘Migration with Dignity’ concept to enhance the adaptive capacity of livelihoods. By using the Sustainable Livelihood Approach this thesis examines what impacts climate change has on different aspects of livelihoods in Kiribati. This study uses a case study approach. Data has been collected through 14 semi-structured interviews during an eight weeks long minor field study on the capital atoll South Tarawa. While Kiribati faces many development challenges, being a least developed country with a rent-based economy, climate change puts additional strains on the country’s capacities to cope with the increasing monetization and urbanisation, and abilities to satisfy the growing population’s aspirations. The empirical evidence shows a need among the population to find education and skilled wage employment. -
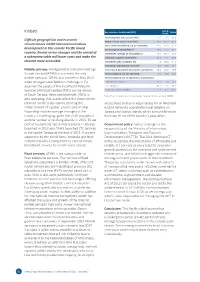
Kiribati Key Indicators for Kiribati (2017) World Pacific Fixed-Telephone Sub
Asia & Kiribati Key indicators for Kiribati (2017) World Pacific Fixed-telephone sub. per 100 inhab. 0�7 9�5 13�0 Difficult geographical and economic Mobile-cellular sub. per 100 inhab. 39�6 104�0 103�6 circumstances inhibit telecommunications Active mobile-broadband sub. per 100 inhab. 42�0 60�3 61�9 development in this remote Pacific island 3G coverage (% of population) 48�0 91�3 87�9 country. Recent sector changes and the arrival of LTE/WiMAX coverage (% of population) 45�0 86�9 76�3 a submarine cable will lower costs and make the Individuals using the Internet (%) 14.6 44�3 48�6 Internet more accessible. Households with a computer (%) 7.3 38�9 47�1 Households with Internet access (%) 6.9 49�0 54�7 Mobile services: Amalgamated Telecoms Holdings International bandwidth per Internet user (kbit/s) 18�9 61�7 76�6 Kiribati Limited (ATHKL) is currently the only Fixed-broadband sub. per 100 inhab. 0�1 13�0 13�6 mobile operator. ATHKL was created in May 2015 Fixed-broadband sub. by speed tiers, % distribution when Amalgamated Telecoms Holdings of Fiji -256 kbit/s to 2 Mbit/s 13�2 2�4 4�2 acquired the assets of the incumbent Telecom -2 to 10 Mbit/s 85�5 7�6 13�2 Services of Kiribati Limited (TSKL) on the islands -equal to or above 10 Mbit/s 1�3 90�0 82�6 of South Tarawa, Betio and Kiritimati. ATHKL is Note: Data in italics are ITU estimates. Source: ITU (as of June 2018). -

SAINT LUCIA Third National Report
SAINT LUCIA Third national report CONTENTS A. REPORTING PARTY ........................................................................................................................ 2 Information on the preparation of the report............................................................................. 2 B. PRIORITY SETTING, TARGETS AND OBSTACLES............................................................................ 6 Priority Setting......................................................................................................................... 8 Challenges and Obstacles to Implementation............................................................................ 9 2010 Target........................................................................................................................... 12 Global Strategy for Plant Conservation (GSPC)........................................................................ 56 Ecosystem Approach .............................................................................................................. 79 C. ARTICLES OF THE CONVENTION.................................................................................................. 81 Article 5 – Cooperation........................................................................................................... 81 Article 6 - General measures for conservation and sustainable use.......................................... 83 Biodiversity and Climate Change...................................................................................... -

Chapter 4 the Marshall Islands, the Federated States of Micronesia and Palau
Chapter 4 The Marshall Islands, the Federated States of Micronesia and Palau By Anirban Biswas A. Brief overview of the Asia-Pacific Trade Agreement During the past decade the intraregional share of total Asia-Pacific exports increased from 44% in 2000 to 52% in 2013, with developing Asia-Pacific countries making the highest contribution.1 In this regard, regional trade agreements such as APTA could be a very good platform for expanding intraregional trade under the agreement. As APTA provides a good platform for South-South trade and has provisions for special and differential treatment for small Pacific Island countries with special needs, such as Micronesian Trade and Economic Community (MTEC) member countries – the Federated States of Micronesia (hereinafter referred to as Micronesia), the Marshall Islands and Palau, these three countries can reap the benefits from increasing trade in the region by joining APTA.2 This section discusses briefly how the study objective was conceived and provides an overview of trade among the Participating States of APTA. It also sets the objective, and discusses the methodology and data sources related to the study. In section B, the economic background, geographical condition, historical/political aspects and external trade structures as well as different preferential trade agreements of the Marshall Islands, Micronesia and Palau are discussed. Section C evaluates the export potential for the Marshall Islands, Micronesia and Palau by examining their exports under the existing concessions given by the Participating States of APTA (covering more than 10,000 items in the Fourth Round). The export potential at 6-digit Harmonized Commodity Description and Coding System (HS) level has been identified. -

New Zealand CJPME Foundation – Human Rights Report Series Published August, 2018
Middle East - New Zealand CJPME Foundation – Human Rights Report Series Published August, 2018 New Zealand - Human Rights Scorecard 1 New Zealand is an island country in the Southern Pacific Ocean east of Australia..2 The country received independence from the British Empire in 1947, though it remains part of the British commonwealth. New Zealand is a highly developed democracy that ranks towards the top in education and quality of life. The indigenous Māori and other Polynesian ethnic groups continue to comprise a large portion of the population, and New Zealand has undergone a revivalist movement to restore rights and cultural empowerment to the colonized indigenous populations. Official language: English, Māori, NZ Sign Language Ethnic groups: 74.0% White European; 14.9% Māori; 11.8% Asian; 7.4% Pacific peoples; 2.9% Other Government: Unitary parliamentary constitutional monarchy - Monarch: Elizabeth II - Prime Minister: Jacinda Ardern - Governor-General: Dame Patsy Reddy Population: 4.9 million Life expectancy: 81 years Under-5 mortality: 6 per 1000 Adult literacy: 99% Death penalty: Abolished Section 1: Overall Development The overall development of a country – considering education, health, income, and other factors – is a strong indicator of whether average citizens have a reasonable chance to enjoy social and economic well-being and mobility. Human Development (UNDP Human Development Index (HDI)) 3 0.915 – Very High Human Development 4 According to the UNDP, New Zealanders enjoyed a gross national income per capita of $32,870, could expect to have on average 12.5 years of schooling. Compared to other countries with similar gross national income per capita, this is a very high HDI. -

Memo Provided By: Globaledge.Msu.Edu and EXPORT.GOV
Cyprus Introduction Key Economic Facts Risk Assessment (Provided by Coface) Cyprus is an island country in the Middle East in the Eastern Income Level (by per capita High Income Country rating: A4 - A somewhat shaky political and Mediterranean Sea. Nearby countries include Turkey, Syria, GNI): economic outlook and a relatively volatile business and Lebanon. Geographically, Cyprus is a central plain with Level of Development: Developed environment can affect corporate payment behavior. mountains to the north and south. The GDP, PPP (current international $36.95 billion (2019) government system is a republic; the chief $): Corporate default probability is still acceptable on average. of state and head of government is the GDP growth (annual %): 3.08% (2019) Business Climate rating: A3 - The business environment president. Cyprus has a market economy GDP per capita, PPP (current $41,254.40 (2019) is relatively good. Although not always available, corporate system in which the prices of goods and international $): financial information is usually reliable. Debt collection and services are determined in a free price system. Cyprus is a Manufacturing, value added (% 5.38% (2019) the institutional framework may have some shortcomings. member of the European Union (EU). of GDP): Current account balance (BoP, -$1.65 billion (2019) Intercompany transactions may run into occasional current US$): difficulties in the otherwise secure environments rated A3. Inflation, consumer prices 0.25% (2019) Strengths (annual %): • Central geographical location -

The European Union and Latin America and the Caribbean Convergent and Sustainable Strategies in the Current Global Environment
The European Union and Latin America and the Caribbean Convergent and sustainable strategies in the current global environment FOR SUSTAINABLE DEVELOPMENT WITH EQUALITY Thank you for your interest in this ECLAC publication ECLAC Publications Please register if you would like to receive information on our editorial products and activities. When you register, you may specify your particular areas of interest and you will gain access to our products in other formats. www.cepal.org/en/suscripciones Alicia Bárcena Executive Secretary Mario Cimoli Deputy Executive Secretary Raúl García-Buchaca Deputy Executive Secretary for Management and Programme Analysis Ricardo Pérez Chief, Publications and Web Services Division This document was prepared by the Economic Commission for Latin America and the Caribbean (ECLAC) for the Meeting of Foreign Ministers of the Community of Latin American and Caribbean States (CELAC) and the European Union, held in Brussels on 16 and 17 July 2018. Álvaro Calderón and Sebastián Rovira of the Division of Production, Productivity and Management of ECLAC were responsible for the overall coordination of the document. The following staff members contributed to its preparation: Leandro Cabello, Mathilde Closset, Marco Dini, Valeria Jordán, Jorge Patiño, Wilson Peres, Cecilia Plottier, Laura Poveda, Nunzia Saporito and Giovanni Stumpo of the Division of Production, Productivity and Management; Daniel Titelman, Jürgen Weller and Cecilia Vera of the Economic Development Division; Sebastián Herreros and Javier Meneses of the Division of International Trade and Integration; Simone Cecchini, Beatriz Morales and Daniela Trucco of the Social Development Division; Eduardo Alatorre, David Barrio Lamarche and Carlos de Miguel of the Sustainable Development and Human Settlements Division; and Jeannette Sánchez of the Natural Resources and Infrastructure Division.