SIROS Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2017 Annual Report 1 I Sirtex 2017 Highlights
2017 ANNUAL REPORT 1 I SIRTEX 2017 HIGHLIGHTS DOSES SOLD 2017 12,578 14,000 12,000 10,000 8,000 6,000 4,000 2,000 0 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 CONTENTS ABOUT SIRTEX 03 2017 FINANCIAL SUMMARY Sirtex Medical Limited is an Australian- 05 CHAIRMAN’S REPORT based global healthcare business working to 09 CHIEF EXECUTIVE OFFICER’S REPORT improve outcomes for people with cancer. 18 ENVIRONMENTAL, SOCIAL AND Our lead product is a targeted radiation GOVERNANCE therapy known as SIR-Spheres® Y-90 resin 22 BOARD OF DIRECTORS microspheres. It is available in more than 23 KEY MANAGEMENT PERSONNEL 40 countries, within over 1,090 certified hospitals to treat patients with 24 FINANCIAL REPORT inoperable liver cancer. Our business revolves around helping medical professionals understand and use our product to improve clinical outcomes and the quality of life for people with liver cancer. While at the same time, we work closely with government and private payers to ensure our patients receive the appropriate reimbursement for our product. We are challenging established practices and developing innovative new therapies that promise to improve the health and lives of many people suffering from cancer or other diseases. Our ongoing success is based on a commitment to serving our customers, professionalism, continuous improvement and innovation. ANNUAL REPORT 2017 I 2 DOSE SALES 12,578 +5.4% REVENUE $234.3m +0.8% NET LOSS AFTER TAX $26.3m -149.0% THE AMERICAS EUROPE, MIDDLE ASIA PACIFIC EAST, AFRICA Boston, United States Frankfurt, -

Australian Listed Equities: Weekly Share Market Wrap
Australian Listed Equities: Weekly Share Market Wrap Total Shareholder Returns as at 15 April 2016 Price 1 week 1 month 6 months 1 year 2 years 3 years 5 years 10 years 10 years Ticker Stock Name $ % % % % % p.a. % p.a. % p.a. % p.a. ranking A2M The A2 Milk Company 1.665 -4.31 2.78 134.51 208.33 - - - - - ABC Adelaide Brighton 5.10 -1.16 6.03 26.15 17.04 19.09 19.37 12.82 10.84 34 ABP Abacus Property Group 3.10 2.99 5.08 -0.47 12.76 20.70 17.15 11.90 -1.96 105 AFI Australian Foundat. 5.53 0.18 -1.60 -4.25 -7.23 0.42 5.09 6.71 5.22 58 AGL AGL Energy Limited. 18.24 -0.65 0.55 15.71 25.25 14.52 10.63 9.90 6.32 53 AHG Automotive Holdings. 3.85 -0.52 -3.78 -1.13 -2.98 4.35 4.94 12.70 14.45 22 AIA Auckland Internation 5.75 -1.88 0.45 25.03 28.95 28.14 37.37 30.93 14.31 23 AIO Asciano Limited 8.84 1.14 -1.67 13.83 38.00 31.68 21.04 13.79 - - AIZ Air New Zealand 2.70 -0.37 9.76 4.19 3.16 27.56 39.16 31.16 13.06 24 ALL Aristocrat Leisure 10.12 4.01 1.50 14.85 23.98 45.00 42.39 29.35 -1.72 102 ALQ ALS Limited 4.38 15.57 1.15 -16.29 -1.83 -16.66 -16.64 -6.48 10.25 37 AMC Amcor Limited 14.76 1.17 4.53 15.59 5.97 25.73 23.23 20.98 11.71 29 AMP AMP Limited 5.79 5.46 2.84 3.13 -8.72 11.19 8.68 4.71 1.17 88 ANN Ansell Limited 19.68 13.30 14.42 3.31 -32.90 7.34 12.16 9.27 7.39 47 ANZ ANZ Banking Grp Ltd 23.85 7.05 -6.07 -13.17 -28.60 -9.52 0.33 6.22 3.50 72 AOG Aveo Group 3.30 0.30 4.10 16.20 24.07 30.19 38.74 -1.59 -9.34 119 APA APA Group 8.71 0.46 5.83 1.14 -1.03 22.97 17.57 21.10 11.78 28 APE AP Eagers Limited 10.00 4.06 -0.58 0.99 24.34 45.68 30.49 -
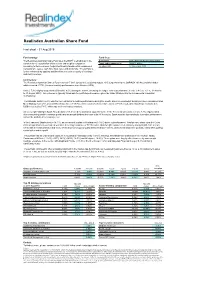
Realindex Australian Share Fund
Realindex Australian Share Fund Fact sheet - 31 Aug 2015 Fund strategy Fund facts The Realindex Australian Share Fund uses the RAFI® methodology in the Benchmark S&P / ASX 200 Accumulation Index construction of its portfolios which selects and weights companies Funds under management $2800.1 million according to their economic footprint defined by fundamental measures of Inception Date 17 November 2008 company size (sales, cash flow, book value and dividends). The portfolio is further enhanced by applying additional factors such as quality of earnings and debt coverage. Commentary The Realindex Australian Shares Fund returned -7.39% (gross of fees) during August 2015 outperforming the S&P/ASX 200 Accumulation Index which returned -7.79% (its worst monthly performance since October 2008). Value (-7.3%) slightly outperformed Growth (-8.0%) during the month, extending the longer-term outperformance trend (-2.4% vs. -6.1%, 12 months to 31 August 2015). This outcome is typically favourable for portfolio performance, given the Value tilt inherent in the Fundamental Indexation methodology. The Materials sector (-5.2%) was the top contributor to relative performance during the month, driven by overweight holdings in steel companies Sims Metal Management (+21.4%) and BlueScope Steel (+18.7%). Sims reported a better-than-expected FY15 result, while BlueScope committed to $200m cost out by FY17, which was well received by investors. An overweight holding in Spark New Zealand (+13.0%) led the portfolio’s outperformance in the Telecommunications sector (-8.3%). Spark rallied after announcing positive earnings growth and an annual dividend increase at its FY15 results. Spark was the top contributor to relative performance across the portfolio at a company level. -

Sep Tem B Er
4 201 Quarterly Newsletter September FML S In this quarterly edition we review performance and attribution. We profile GBST Holdings and IProperty Group. Offshore we take a look at QE and US Energy. We also consider how Technology for the Ages changes with each generation. Photo: School holidays - no worries, surfing for a 9 year old maybe as good as it gets and no technology needed. Selector Funds Management Limited ACN 102756347 AFSL 225316 Level 3, 10 Bridge Street Sydney NSW 2000 Australia Tel 612 8090 3612 www.selectorfund.com.au selector About Selector We are a boutique fund manager and we have a combined experience of over 150 years. We believe in long term wealth creation and building lasting relationships with our investors. Our focus is stock selection. Our funds are high conviction, concentrated and index unaware. As a result we have low turnover and produce tax effective returns. First we identify the best business franchises with the best management teams. Then we focus on valuations. Please forward to us contact details if you would like future newsletters to be emailed to family, friends or business colleagues. Selector Funds Management Limited ACN 102756347 AFSL 225316 Level 3, 10 Bridge Street Sydney NSW 2000, Australia Telephone 612 8090 3612 Web www.selectorfund.com.au selector September 2014 Selector High Conviction Equity Fund Quarterly Newsletter #45 Dear Investor, With the company reporting season over for another year, investor attention has quickly shifted to the road ahead. Here the simple message, proceed with caution, has been top of mind and for good reason. -

SIR-Spheres® Y-90 Resin Microspheres
Manufacturer: US Sales Office: Sirtex Medical Limited Sirtex Medical Inc Level 33, 101 Miller Street, 300 Unicorn Park Drive, North Sydney NSW 2060, Australia Woburn MA 01801, USA Tel: +61 2 9964 8400 Tel: 1 888 474 7839 www.sirtex.com 4 Warnings • Some patients may experience gastric problems following treatment but H-2 blocking agents may 4.1 Non-Target Delivery of SIR-Spheres be used the day before implantation of SIR- microspheres Spheres microspheres and continued as needed to reduce gastric complications. Inadvertent delivery of SIR-Spheres microspheres to extra-hepatic structures such as the esophagus, • Many patients may experience abdominal pain SIR-Spheres® Y-90 resin stomach, duodenum, gallbladder or pancreas may immediately after administration of SIR-Spheres result in radiation injury to these structures. microspheres and pain relief may be required. microspheres Meticulous angiographic technique must be • SIR-Spheres microspheres demonstrated a mild (Yttrium-90 microspheres) employed to prevent the non-target delivery of SIR- sensitization potential when tested dermally in an Spheres microspheres to any extra-hepatic animal model. structures. 1 Description 4.2 Radioembolization Induced Liver 6 Clinical Trial Results SIR-Spheres microspheres consist of biocompatible microspheres containing yttrium-90 with a size Disease (REILD) In a randomized, controlled clinical trial, a total of 70 patients were studied in two arms, 34 patients with between 20 and 60 microns in diameter. Yttrium-90 Delivery of excessive radiation to the normal liver FUDR chemotherapy (control group), and 36 is a high-energy pure beta-emitting isotope with no parenchyma may result in REILD – see description patients with FUDR plus SIR-Spheres microspheres. -

Smallco Investment Fund
Smallco Investment Fund Quarterly Update 30 September 2014 SIF Portfolio Commentary SIF Performance Smallco Investment Fund (SIF) delivered 6.8% during the Return to 30 September 2014 September quarter and for the 12 months was up a solid 12.3%. During the quarter Iress, Isentia Group and Sirtex 3 months 6.8% Medical performed positively, while Carsales.com, Ozforex 1 year 12.3% Group and Macquarie Atlas Roads were negative 3 year p.a. 36.4% contributors. 5 year p.a. 22.5% For the September quarter the Small Ordinaries index was 10 year p.a. 13.6% modestly positive at 1.5% while for the year to 30 Since inception (31.10.00) p.a. 14.2% September it was down -0.1%. Value of $100,000 invested at inception $631,173 In general the Australian market had a solid reporting Fund returns calculated after all fees and expenses and based season and that was reflected in share prices during upon exit price and reinvestment of distributions. August, particularly for Industrial companies. However, towards the end of the quarter, the Australian and international markets became concerned about global political tensions – Ukraine, Iraq and Hong Kong – with a falling iron ore price having a significant negative impact on the Australian resources index. SIF Structure: by sector (look through) One of our major holdings is IRESS (IRE). SIF originally invested in IRE in 2002 when it had a market capitalisation of $260m. At the time we invested it was already the dominant provider of information systems to the professional equity market in Australia and New Zealand (ANZ). -

2020 Mid-Year Report
NON-U.S. LITIGATION UPDATE / 2020 MID-YEAR REPORT The LIAISON NEW YORK | WASHINGTON, D.C. | DELAWARE LABATON.COM Contents and EXECUTIVE SUMMARY EXECUTIVE SUMMARY CONTENTS Labaton Sucharow is pleased to present The Liaison: 2020 Mid-Year Report. P 1 EXECUTIVE SUMMARY The Firm has been a pioneer in protecting clients’ interests in non- U.S. litigation. With its nearly 20 years of experience abroad, and deep P 2 TRENDS IN NON-U.S. SECURITIES CLASS relationships with law firms around the world, Labaton Sucharow ACTION LITIGATION has a unique perspective on investment-related issues and recovery opportunities outside the United States. Featured in this edition are: P 4 NOTEWORTHY DEVELOPMENTS P 4 Changes to the Landscape for • Recent settlements in non-U.S. securities matters; Securities Class Action in Australia • Noteworthy developments regarding securities litigation in Australia; P 7 A New Opportunity for U.S. Investors • Ramifications of the recent U.S. decision in the Toshiba class action; to Seek Recovery in the United States • Summary of the newly-proposed Westpac action in Australia; or Losses on Shares Abroad? • Updates on select pending non-U.S. securities actions; and • Deadlines for participation in non-U.S. actions and for the submission P 10 NEWLY ANNOUNCED MATTER: of settlement claims. WESTPAC BANKING CORPORATION We would be happy to provide more comprehensive assessments and P 11 ONGOING CASE STUDIES recommendations with regard to any of the topics discussed or highlighted P 12 Germany in The Liaison. P 12 The Netherlands P 13 United Kingdom With best regards, P 14 Denmark P 14 New Zealand Labaton Sucharow LLP P 15 Australia P 17 Japan P 18 Brazil P 19 CONTACT US The LIAISONPAGE 1 THE LIAISON Trends in Non-U.S. -

TOP KEY BOARD and EXECUTIVE MOVEMENTS in AUSTRALIA 18Th – 22Nd SEPTEMBER 2017
TOP KEY BOARD AND EXECUTIVE MOVEMENTS IN AUSTRALIA 18th – 22nd SEPTEMBER 2017 Air New Zealand Ltd: Jeff McDowall appointed as Chief Financial Officer, effective as at 1 January 2018. Rob McDonald will be leaving the company at the end of the year. Nick Judd has been appointed as Chief Strategy, Networks and Alliances Officer replacing Stephen Jones and commences his new role on October 2 2017. Altura Mining Ltd: Chris Evans has been appointed as Chief Operating Officer commencing on 20 September 2017. Australian Ethical Investment Ltd: Michael Monaghan has been appointed as a Non- Executive Director, effective 22 September 2017. Australian Unity Ltd: Esther Kerr-Smith has been appointed as Group Executive, Finance and Strategy with effect from 25 September 2017. Challenger Ltd: Brenda Shanahan has advised that she will resign as a Non-Executive Director of Challenger at the Annual General Meeting on 26 October 2017. CIMIC Group Ltd: Kate Spargo has been appointed as a Non-Executive Director. Commonwealth Bank of Australia Ltd: Annabel Spring has resigned from her current positon as Group Executive, Wealth Management and will leave the Company in December 2017. Michael Venter, current Chief Financial Officer, International Financial Services has been appointed Chief Operating Officer, Wealth Management, effective 21 September 2017. Fletcher Building Ltd: John Judge and Kate Spargo have both resigned as Non- Executive Directors, with John leaving at the Annual General Meeting on 25 October 2017 and Kate Spargo on 20 September 2017. Fletcher Building Ltd: Kate Daly, Chief People and Communications Officer, has resigned. Fonterra Co-operative Group Ltd: Marc Rivers has been appointed as Chief Financial Officer, effective 1 March 2018. -

Litigation Funding and the Regulation of the Class Action Industry Report
Parliamentary Joint Committee on Corporations and Financial Services Litigation funding and the regulation of the class action industry December 2020 © Commonwealth of Australia ISBN 978-1-76093-169-8 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The details of this licence are available on the Creative Commons website: https://creativecommons.org/licenses/by-nc-nd/4.0/. Printed by the Senate Printing Unit, Parliament House, Canberra. Committee membership Chair Senator James Paterson LP, VIC Deputy Chair Mr Steve Georganas MP ALP, SA Members Senator Andrew Bragg LP, NSW Mr Jason Falinski MP LP, NSW Mr Patrick Gorman MP ALP, WA Ms Celia Hammond MP LP, WA Senator Deborah O'Neill ALP, NSW Senator Louise Pratt ALP, WA Mr Bert van Manen MP LP, QLD Senator Nick McKim AG, TAS (from 6 October 2020) Former Members Senator Peter Whish-Wilson AG, TAS (until 6 October 2020) Secretariat Dr Patrick Hodder, Committee Secretary Dr Jon Bell, Principal Research Officer Ms Emily Treeby, Senior Research Officer Ms Eleonora Fionga, Administrative Officer Suite SG.64 Telephone: +61 2 6277 3583 PO Box 6100 Fax: +61 2 6277 5719 Parliament House Email: [email protected] Canberra ACT 2600 Website: www.aph.gov.au/joint_corporations iii Contents Committee membership ...................................................................................................................iii Abbreviations ................................................................................................................................... -

Introduction
INTRODUCTION CONTRIBUTNG ORGANISATIONS CONTRIBUTORS LISTED ALPHABETICALLY 7-Eleven Stores Amgen Australia ABB Australia AMT GROUP Abigroup Amway of Australia Accolade Wines Anglican Retirement Villages Diocese of Sydney Actavis Australia AP Moller-Maersk A/S Actelion Pharmaceuticals APL Adelaide Brighton APT Management Services (APA Group) Adidas Australia Arch Wood Protection (Aust) AECOM Australia Arrow Electronics Australia Afton Chemical Asia Pacific LLC Ashland Hercules Water Technologies (Australia) AGC Aspen Pharmacare Australia Aggreko Australia AstraZeneca AGL Energy ATCO Australia Aimia Atlassian Air Liquide Australia Aurecon Group Alcon Laboratories Australia Ausenco Alfa Laval Australia Ausgrid (formerly EnergyAustralia) Allergan Australia Aussie Home Loans Allied Mills Australand Property Group Alpha Flight Services Australia Post Alphapharm Australian Agricultural Company Alstom Australian Catholic University Altium Australian Institute of Company Directors Amadeus IT Pacific Australian Pharmaceutical Industries (Priceline, Soul Pattinson Chemist) Ambulance Victoria Australian Prudential Regulation Authority Amcor Australian Timken Propriety AMEC Mining & Metals Australian Turf Club © 2012 Mercer Consulting (Australia) Pty Ltd Top Management Remuneration Report 1.7 INTRODUCTION Avant Mutual Group BMW MELBOURNE Avery Dennison BMW Sydney AVJennings Boart Longyear Avon Cosmetics BOC Baptist Community Services NSW & ACT Boehringer Ingelheim Barbeques Galore Boeing Defense Australia BASF Australia Bombardier Transportation Australia -

Weekly Share Market Wrap
Australian Listed Equities: Weekly Share Market Wrap Total Shareholder Returns as at 25 September 2015 Price 1 week 1 month 6 months 1 year 2 years 3 years 5 years 10 years 10 years Ticker Stock Name $ % % % % % p.a. % p.a. % p.a. % p.a. ranking ABC Adelaide Brighton Ltd 4.40 -2.00 0.45 -0.89 38.64 15.79 17.95 9.65 11.04 36 ABP Abacus Property Group 3.13 -1.26 3.30 9.35 28.39 23.55 22.66 13.61 -0.78 100 AFI Australian Foundat. 5.73 -2.05 -5.29 -6.68 -0.83 3.49 13.30 7.73 6.84 54 AGL AGL Energy Ltd. 16.20 -0.98 -0.98 11.61 22.74 8.66 9.08 5.27 - - AHD Amalgamated Holdings 12.96 -2.19 2.40 14.52 42.66 29.87 28.03 20.99 14.71 22 AHG Automotive Holdings. 3.95 -0.50 -1.92 -3.55 10.61 5.17 15.92 16.47 - - AHY Asaleo Care Ltd 1.76 0.57 16.88 -3.74 -4.68 - - - - - AIA Auckland Internation 4.54 2.71 1.34 3.18 37.45 27.46 32.59 25.95 10.53 38 AIO Asciano Ltd 8.37 -0.95 -0.59 27.40 36.55 19.69 25.85 12.65 - - AIZ Air New Zealand Ltd 2.25 1.81 -2.27 -11.19 34.72 42.66 44.30 22.42 11.64 31 ALL Aristocrat Leisure 8.61 2.14 4.11 5.21 49.15 40.83 48.55 20.20 -1.25 102 ALQ ALS Ltd 4.67 -5.47 -2.10 -5.17 -14.69 -28.01 -11.72 -0.04 14.93 21 AMC Amcor Ltd 12.89 -1.30 0.96 -8.18 18.76 19.57 27.01 19.72 11.24 34 AMP AMP Ltd 5.55 -4.15 -4.21 -14.82 2.92 13.48 13.00 6.18 2.61 87 ANN Ansell Ltd 19.90 0.30 -3.63 -25.96 5.08 0.94 11.07 11.05 7.55 50 ANZ ANZ Banking Grp Ltd 27.00 -4.93 -3.54 -25.09 -8.01 -1.38 9.30 8.12 5.85 59 AOG Aveo Group 2.67 -0.37 -3.26 -1.45 27.10 55.47 21.18 -5.22 -8.27 117 APA APA Group 8.68 2.97 1.88 -0.39 25.34 27.86 28.72 22.71 13.69 -

STOXX Pacific 100 Last Updated: 01.08.2017
STOXX Pacific 100 Last Updated: 01.08.2017 Rank Rank (PREVIOU ISIN Sedol RIC Int.Key Company Name Country Currency Component FF Mcap (BEUR) (FINAL) S) AU000000CBA7 6215035 CBA.AX 621503 Commonwealth Bank of Australia AU AUD Y 98.1 1 1 AU000000WBC1 6076146 WBC.AX 607614 Westpac Banking Corp. AU AUD Y 72.3 2 2 AU000000ANZ3 6065586 ANZ.AX 606558 Australia & New Zealand Bankin AU AUD Y 58.9 3 3 AU000000BHP4 6144690 BHP.AX 614469 BHP Billiton Ltd. AU AUD Y 56.2 4 5 AU000000NAB4 6624608 NAB.AX 662460 National Australia Bank Ltd. AU AUD Y 54.3 5 4 AU000000CSL8 6185495 CSL.AX 618549 CSL Ltd. AU AUD Y 38.8 6 6 AU000000TLS2 6087289 TLS.AX 608545 Telstra Corp. Ltd. AU AUD Y 33.0 7 7 AU000000WES1 6948836 WES.AX 694883 Wesfarmers Ltd. AU AUD Y 31.3 8 8 AU000000WOW2 6981239 WOW.AX 698123 Woolworths Ltd. AU AUD Y 23.4 9 9 AU000000RIO1 6220103 RIO.AX 622010 Rio Tinto Ltd. AU AUD Y 18.9 10 11 AU000000MQG1 B28YTC2 MQG.AX 655135 Macquarie Group Ltd. AU AUD Y 18.6 11 10 AU000000TCL6 6200882 TCL.AX 689933 Transurban Group AU AUD Y 15.9 12 12 AU000000SCG8 BLZH0Z7 SCG.AX AU01Z4 SCENTRE GROUP AU AUD Y 14.9 13 14 AU000000WPL2 6979728 WPL.AX 697972 Woodside Petroleum Ltd. AU AUD Y 14.4 14 13 AU000000SUN6 6585084 SUN.AX 658508 SUNCORP GROUP LTD. AU AUD Y 12.5 15 15 AU000000AMC4 6066608 AMC.AX 606660 Amcor Ltd. AU AUD Y 12.0 16 16 AU000000QBE9 6715740 QBE.AX 671574 QBE Insurance Group Ltd.