265 M. Lucioni, Practical Guide to Neck Dissection, DOI 10.1007/978
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Importance of the Middle Meningeal Artery
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Jagiellonian Univeristy Repository FOLIA MEDICA CRACOVIENSIA 41 Vol. LIII, 1, 2013: 41–46 PL ISSN 0015-5616 Przemysław Chmielewski1, Janusz skrzat1, Jerzy waloCha1 CLINICAL IMPORTANCE OF THE MIDDLE MENINGEAL ARTERY Abstract: Middle meningeal artery (MMA)is an important branch which supplies among others cranial dura mater. It directly attaches to the cranial bones (is incorporated into periosteal layer of dura mater), favors common injuries in course of head trauma. This review describes available data on the MMA considering its varability, or treats specific diseases or injuries where the course of MMA may have clinical impact. Key words: Middle meningeal artery (MMA), aneurysm of the middle meningeal artery, epidural he- matoma, anatomical variation of MMA. TOPOGRAPHY OF THE MIDDLE MENINGEAL ARTERY AND ITS BRANCHES Middle meningeal artery (MMA) [1] is most commonly the strongest branch of maxillary artery (from external carotid artery) [2]. It supplies blood to cranial dura mater, and through the numerous perforating branches it nourishes also periosteum of the inner aspect of cranial bones. It enters the middle cranial fossa through the foramen spinosum, and courses between the dura mater and the inner aspect of the vault of the skull. Next it divides into two terminal branches — frontal (anterior) which supplies blood to bones forming anterior cranial fossa and the anterior part of the middle cranial fossa; parietal branch (posterior), which runs more horizontally toward the back and supplies posterior part of the middle cranial fossa and supratentorial part of the posterior cranial fossa. -

Morphometric Analysis of Stylomastoid Foramen Location and Its Clinical Importance
Dental Communication Biosc.Biotech.Res.Comm. Special Issue Vol 13 No 8 2020 Pp-108-111 Morphometric Analysis of Stylomastoid Foramen Location and its Clinical Importance Hemanth Ragav N V1 and Yuvaraj Babu K2* 1Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai- 600077, India 2Assistant Professor, Department of Anatomy, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Science, Saveetha University, Chennai- 600077, India ABSTRACT The stylomastoid foramen is located between the styloid process and mastoid process of the temporal bone. Facial nerve and Stylomastoid branch of posterior auricular artery passes through this stylomastoid foramen. The facial nerve can be blocked at this stylomastoid foramen but it has high risk of nerve damage. For Nadbath facial nerve block, stylomastoid foramen is the most important site. Facial canal ends at this foramen and it is the important motor portion of this stylomastoid foramen. A total of 50 dry skulls from the Anatomy Department of Saveetha Dental College were studied to locate the position of the centre of the stylomastoid foramen with respect to the tip of mastoid process and the articular tubercle of the zygomatic arch by a digital vernier caliper. All measurements were tabulated and statistically analysed. In our study, we found the mean distance of stylomastoid foramen from mastoid processes 16.31+2.37 mm and 16.01+2.08 mm on right and left. Their range is 10.48-23.34 mm and 11.5-21.7 mm. The mean distance of stylomastoid foramen from articular tubercle is 29.48+1.91 mm and 29.90+1.62 mm on right and left. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Clinical Importance of the Middle Meningeal Artery
FOLIA MEDICA CRACOVIENSIA 41 Vol. LIII, 1, 2013: 41–46 PL ISSN 0015-5616 Przemysław Chmielewski1, Janusz skrzat1, Jerzy waloCha1 CLINICAL IMPORTANCE OF THE MIDDLE MENINGEAL ARTERY Abstract: Middle meningeal artery (MMA)is an important branch which supplies among others cranial dura mater. It directly attaches to the cranial bones (is incorporated into periosteal layer of dura mater), favors common injuries in course of head trauma. This review describes available data on the MMA considering its varability, or treats specific diseases or injuries where the course of MMA may have clinical impact. Key words: Middle meningeal artery (MMA), aneurysm of the middle meningeal artery, epidural he- matoma, anatomical variation of MMA. TOPOGRAPHY OF THE MIDDLE MENINGEAL ARTERY AND ITS BRANCHES Middle meningeal artery (MMA) [1] is most commonly the strongest branch of maxillary artery (from external carotid artery) [2]. It supplies blood to cranial dura mater, and through the numerous perforating branches it nourishes also periosteum of the inner aspect of cranial bones. It enters the middle cranial fossa through the foramen spinosum, and courses between the dura mater and the inner aspect of the vault of the skull. Next it divides into two terminal branches — frontal (anterior) which supplies blood to bones forming anterior cranial fossa and the anterior part of the middle cranial fossa; parietal branch (posterior), which runs more horizontally toward the back and supplies posterior part of the middle cranial fossa and supratentorial part of the posterior cranial fossa. Branches of MMA traverse the arterial grooves giving rise to abundant lateral tracts which supply dura mater and periosteum. -

Variations in the Morphology of Stylomastoid Foramen: a Possible Solution to the Conundrum of Unexplained Cases of Bell’S Palsy S.K
Folia Morphol. Vol. 80, No. 1, pp. 97–105 DOI: 10.5603/FM.a2020.0019 O R I G I N A L A R T I C L E Copyright © 2021 Via Medica ISSN 0015–5659 eISSN 1644–3284 journals.viamedica.pl Variations in the morphology of stylomastoid foramen: a possible solution to the conundrum of unexplained cases of Bell’s palsy S.K. Ghosh , R.K. Narayan Department of Anatomy, All India Institute of Medical Sciences, Phulwarisharif, Patna, Bihar, India [Received: 12 January 2020; Accepted: 2 February 2020] Background: Stylomastoid foramen is the terminal part of facial canal and is the exit gateway for facial nerve from skull base. We hypothesized that anatomical variations of this foramen could be a risk factor for the injury of facial nerve re- sulting in unilateral facial nerve paralysis or Bell’s palsy. Hence the present study was conducted to study the variations in size and shape of stylomastoid foramen in dry adult human skulls. Materials and methods: The study was conducted on 37 dry adult human skulls of unknown age and sex. High resolution images of the skulls under study were processed by ImageJ software and observations were undertaken. Results: Total eight variations of stylomastoid foramen were observed in terms of shape. The common variants were round, oval and square (present in 83.79% skulls on right side and 81.07% skulls on left side), whereas the rare variants were triangular, rectangular, serrated, bean-shaped and irregular. It was noted that stylomastoid foramen were associated with extensions (45.95% skulls) and also adjacent foramen (18.92% skulls). -

Cubical Anatomy of Several Ducts and Vessels by Injection Methods of Acrylic Resin V
Cubical Anatomy of Several Ducts and Vessels by Injection Methods of Acrylic Resin V. Arterial distribution of the temporal muscle in some mammals By Tokuo Fujimoto Department of Anatomy, Osaka Dental College, Osaka (Director : Prof. Y. Tani g u c h i) (With 33 figures in 10 plates and one table.) Argument of this research was announced at 64th Annual Session of the Japanese Association of Anatomists, March, 1959, Tokyo. Preface On carotid arteries and their ramifications, anthropological and comparative anatomical or other works have been abundantly seen. But, those on the arterial supply for the muscle of the head and neck, from a different new point, are few. Especially no observation on the finer vascular distribution in the individual muscle has been made. The author has here undertaken cubical comparative anatomical study on blood supplying routes for temporal muscles of mammals easy to obtain and of human fetuses, by corrosion specimens of acrylic resin. Diverging features of all arteries from the external carotid or its branches taking part in this muscle, ramifications and anastomoses in the inner or outer part of the muscle, and distribut- ing territories of each branch in the muscle, are observed. The tem- poral muscle as well as the masseter, being rich in red muscle fibres, play a strong (dynamically) and important role in the mastication, and it is thought to be significant to throw light on the arterial distribution of them. Although, many comparative results of the carotid arterial system in mammals have been published, they can 389 390 Tokuo Fujimoto not be said to be faultless and perfect on the deeper part. -

Microsurgical Anatomy of the Dural Arteries
ANATOMIC REPORT MICROSURGICAL ANATOMY OF THE DURAL ARTERIES Carolina Martins, M.D. OBJECTIVE: The objective was to examine the microsurgical anatomy basic to the Department of Neurological microsurgical and endovascular management of lesions involving the dural arteries. Surgery, University of Florida, Gainesville, Florida METHODS: Adult cadaveric heads and skulls were examined using the magnification provided by the surgical microscope to define the origin, course, and distribution of Alexandre Yasuda, M.D. the individual dural arteries. Department of Neurological RESULTS: The pattern of arterial supply of the dura covering the cranial base is more Surgery, University of Florida, complex than over the cerebral convexity. The internal carotid system supplies the Gainesville, Florida midline dura of the anterior and middle fossae and the anterior limit of the posterior Alvaro Campero, M.D. fossa; the external carotid system supplies the lateral segment of the three cranial Department of Neurological fossae; and the vertebrobasilar system supplies the midline structures of the posterior Surgery, University of Florida, fossa and the area of the foramen magnum. Dural territories often have overlapping Gainesville, Florida supply from several sources. Areas supplied from several overlapping sources are the parasellar dura, tentorium, and falx. The tentorium and falx also receive a contribution Arthur J. Ulm, M.D. from the cerebral arteries, making these structures an anastomotic pathway between Department of Neurological Surgery, University of Florida, the dural and parenchymal arteries. A reciprocal relationship, in which the territories Gainesville, Florida of one artery expand if the adjacent arteries are small, is common. CONCLUSION: The carotid and vertebrobasilar arterial systems give rise to multiple Necmettin Tanriover, M.D. -

Topographic Anatomy of the Head
O. V. Korencov, G. F. Tkach TOPOGRAPHIC ANATOMY OF THE HEAD Study guide Ministry of Education and Science of Ukraine Ministry of Health of Ukraine Sumy State University O. V. Korencov, G. F. Tkach TOPOGRAPHIC ANATOMY OF THE HEAD Study guide Recommended by Academic Council of Sumy State University Sumy Sumy State University 2016 УДК 611.91(075.3) ББК 54.54я73 K66 Reviewers: L. V. Phomina – Doctor of Medical Sciences, Professor of Vinnytsia National Medical University named after M. I. Pirogov; M. V. Pogorelov – Doctor of Medical Sciences, Professor of Sumy State University Recommended for publication by Academic Council of Sumy State University as а study guide (minutes № 5 of 11.02.2016) Korencov O. V. K66 Topographic anatomy of the head : study guide / O. V. Korencov, G. F. Tkach. – Sumy : Sumy State University, 2016. – 81 р. ISBN 978-966-657-607-4 This manual is intended for the students of medical higher educational institutions of IV accreditation level, who study Human Anatomy in the English language. Посібник рекомендований для студентів вищих медичних навчальних закладів IV рівня акредитації, які вивчають анатомію людини англійською мовою. УДК 611.91(075.3) ББК 54.54я73 © Korencov O. V., Tkach G. F., 2016 ISBN 978-966-657-607-4 © Sumy State University, 2016 TOPOGRAPHIC ANATOMY OF THE HEAD The head is subdivided into two following departments: the brain and facialohes. They are shared by line from the glabella to the supraorbital edge along the zygomatic arch to the outer ear canal. The brain part consists of fornix and base of the skull. The fornix is divided into fronto- parieto-occipital region, paired temporal and mastoid area. -

Chapter One Introduction
Chapter one Introduction 1 Chapter one 1.1Introduction Examination of the carotid wall gives every clinician an opportunity to evaluate subclinical alterations in wall structure that precede and predict future cardiovascular clinical events. B-mode ultrasonography is a noninvasive, safe, easily performed, reproducible, sensitive, relatively inexpensive and widely available method for detection of early stages of atherosclerosis and is accepted as one of the best methods for evaluation of arterial wall structure(Escardio,2015). High resolution B-mode ultrasonography enables quantitative measurement of the thickness of the intima-media layer of superficial large arteries non-invasively. In this observational study we assess the normal measurement of carotid artery intima-media thickness in Sudanese people and correlate it with age ,genderand body mass index(BMI).Intima-media thickness (IMT), also called intimal medial thickness, is a measurement of the thickness of tunica-intima and tunica-media, the innermost two layers of the wall of an artery. The measurement is usually made by external ultrasound and occasionally by internal, invasive ultrasound catheters.Measurement of normal carotid artery intima-media thickness measurement at various non-modifiable factors are necessary for the detection of abnormal measurement in CAIMT, most was based on western population .to our knowledge there no data on the CAIMT measurement in Sudanese people (Salonen, 1991). In a previous study the range of intima media thickness is from 0.04cm-0.07cm in healthy Sudanese people (Mohammed, 2012). Also another studies intima media thickness of carotid artery was measured by sonographic method in healthy India and Bangladesh. They found that the mean CAIMT for healthy subject including all age group was (754.94±11.96micron) (Jayantaet.al, 2012). -

Fetal Anatomy of the Facial Nerve Trunk and Its Relationship with Posterior Auricular Artery
Surgical and Radiologic Anatomy (2019) 41:153–159 https://doi.org/10.1007/s00276-018-2126-x ORIGINAL ARTICLE Fetal anatomy of the facial nerve trunk and its relationship with posterior auricular artery Özlem Elvan1 · Alev Bobuş2 · Semra Erdoğan3 · Mustafa Aktekin4 · Zeliha Kurtoğlu Olgunus2 Received: 18 May 2018 / Accepted: 22 October 2018 / Published online: 26 October 2018 © Springer-Verlag France SAS, part of Springer Nature 2018 Abstract Purpose The aims of the study are to define anatomy of the facial nerve (FN) and its main trunks as well as their relationship with the posterior auricular artery in fetal period to evaluate the data for regional surgery in newborns and young infants. Methods Formalin-fixed 34 fetuses from anatomy laboratory collection with a mean gestational age of 26.4 ± 4.6 (20–36) weeks were dissected. Parameters regarding the presence of major or minor trunks, width, length, branching pattern of FN were evaluated according to side, gender and trimester. The positional relationship of posterior auricular artery with the FN trunk was inspected. Results On all sides only the major trunk of the FN was detected. For length and width parameters, there was no statistically significant difference for side and gender except for trimester. Linear functions were found as 0.329 + 0.025 × weeks for width and 5.264 + 0.185 × weeks for length. There are statistically significant linear relationships between width and length of the FN trunk and week parameters as r = 0.507, p < 0.001 and r = 0.484, p < 0.001, respectively. Posterior auricular artery crossed FN trunk laterally in 42 of 53 sides, medially in 9 sides while it was puncturing it proximally in 2 sides. -
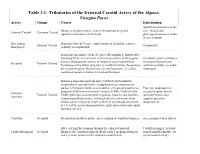
Tributaries of the External Carotid Artery of the Alpaca, Vicugna Pacos
Table 3.1: Tributaries of the External Carotid Artery of the Alpaca, Vicugna Pacos Artery Origin Course Distribution Superficial structures of the Begins at occipital artery; courses throughout deep and face; lingual and External Carotid Common Carotid superficial structures of the head. pharyngeal structures of the deeper cranium Descending Branches from ECA near caudal border of mandible; courses External Carotid Oropharynx Pharyngeal ventrally to oropharynx. From superior surface of ECA, gives off condylar a. shortly after departing ECA; as it ascends, scours deep surface of the jugular Occipital region; terminates process and posterior surface of temporal crest/mastoid bone. in occipital parenchyma Occipital External Carotid Terminates in by bifurcating into 1) smaller branches that perfuse and intracranially as caudal the occipital region (Nuchal muscles and ligament); 2) caudal meningeal meningeal (enters cranium via mastoid foramen) Branches from superolateral aspect of ECA just behind the tympanohyal; ascends between jugular process and posterior surface of tympanic bulla; scores surface of mastoid posterior to Posterior scalp/superior tympanic bulla/external acoustic meatus (EAM). Ventral to the occipital region; rostral Common External Carotid EAM, splits into posterolateral zygomatic branch (also ramifies auricular branch also Auricular temporomandibular joint); stylomastoid artery branches from supplies posterior medial surface of parent vessel at level of stylomastoid foramen; temporalis m. at level of the crista supramastoidea, -

Facial Nerve A73 (1)
FACIAL NERVE A73 (1) Facial Nerve Last updated: April 20, 2019 CEREBELLOPONTINE ANGLE – emerges from brain stem ventrolaterally near posterior aspect of pons, 9.5-14.5 mm from midline and 0.5-2.0 mm medial to where CN8 enters brain stem. – 23-24 mm long and 1-2 mm wide. – runs obliquely (anteriorly and laterally) from pontomedullary sulcus to internal auditory canal. – at times, CN7 axons pass through transverse fibers of middle cerebellar peduncle; – within subarachnoid space, CN7 is joined by CN8, which travels lateral and slightly inferior to CN7. – nervus intermedius runs between CN8 and motor root of CN7. – CN5 is located anteriorly. – in cerebellopontine angle, CN7 has no epineurium (covered only with pia mater). – anterior inferior cerebellar artery is found ventrally (between nerves and pons). INTERNAL AUDITORY MEATUS – meatal segment is 7-8 mm long. – nervus intermedius joins motor root to form common trunk (lies within anterosuperior segment of meatus) - motor fibers are anterior, whereas nervus intermedius fibers remain posteriorly. – at lateral end of meatus: horizontal partition (transverse or falciform crest) separates CN7 from cochlear nerve inferiorly; vertical crest (Bill's bar) separates CN7 from superior vestibular nerve located posteriorly. BLOOD SUPPLY - labyrinthine artery. FACIAL CANAL SEGMENTS: 1) labyrinthine (3-4 mm long) lies within narrowest portion of bony facial canal; passes (forward and downward) between ampulla of superior semicircular canal and cochlea; at geniculate ganglion, nerve turns 75 posteriorly (EXTERNAL GENU); greater superficial petrosal nerve exits 90 anteriorly from geniculate. 2) tympanic (12-13 mm long) continues along medial wall of tympanic cavity, few millimeters medial to incus, superior and posterior to cochleariform process, along upper edge of oval window, inferior to lateral semicircular canal; at origin of stapedius tendon (from pyramidal process), nerve turns 120, continuing inferiorly.