Analysis of Radiator Coolant Mixture Using Ethylene Glycol and Di-Ethylene Glycol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How You Can Become Registered As an Architectural Engineer
PDHonline Course A123 (2 PDH) How You Can Become Registered as an Architectural Engineer Instructor: J. Paul Guyer, P.E., R.A., Fellow ASCE, Fellow AEI 2012 PDH Online | PDH Center 5272 Meadow Estates Drive Fairfax, VA 22030-6658 Phone & Fax: 703-988-0088 www.PDHonline.org www.PDHcenter.com An Approved Continuing Education Provider www.PDHcenter.com PDH Course A123 www.PDHonline.org TABLE OF CONTENTS 1. The Opportunity 3 2. Some Historical Perspective 4 3. This is an Opportunity Not Just for 13 Graduates of Architectural Engineering Programs 4. This is an Opportunity for PEs Already 18 Registered in Other Disciplines 5. The Examination 20 6. Information You Need to Take 26 Into the Exam 7. Career Development 28 © J. Paul Guyer 2009 Page 2 of 29 www.PDHcenter.com PDH Course A123 www.PDHonline.org How You Can Become Registered as an Architectural Engineer J. Paul Guyer, P.E., R.A., Fellow ASCE, Fellow AEI COURSE CONTENT 1. THE OPPORTUNITY This is an opportunity for you…. This is a career enhancing opportunity for engineers interested in the design of buildings and related infrastructure. It is an opportunity for you to take a leadership position in the enterprise of designing and constructing buildings. But first, some background…. As we all know, the undertaking of design of buildings and related infrastructure is one of the most multidisciplinary activities in which engineers engage. It requires the skills, efforts and involvement of structural engineers, civil engineers, mechanical engineers, electrical engineers, fire protection engineers and traditionally trained architects. However….there has never existed a “platform” to bring these diverse skills and capabilities together in order to deliver coordinated services to the clients, companies and agencies needing a cost effective and efficient mechanism to deliver the buildings and related infrastructure they need. -

MSME with Depth in Fluid Mechanics
MSME with depth in Fluid Mechanics The primary areas of fluid mechanics research at Michigan State University's Mechanical Engineering program are in developing computational methods for the prediction of complex flows, in devising experimental methods of measurement, and in applying them to improve understanding of fluid-flow phenomena. Theoretical fluid dynamics courses provide a foundation for this research as well as for related studies in areas such as combustion, heat transfer, thermal power engineering, materials processing, bioengineering and in aspects of manufacturing engineering. MS Track for Fluid Mechanics The MSME degree program for fluid mechanics is based around two graduate-level foundation courses offered through the Department of Mechanical Engineering (ME). These courses are ME 830 Fluid Mechanics I Fall ME 840 Computational Fluid Mechanics and Heat Transfer Spring The ME 830 course is the basic graduate level course in the continuum theory of fluid mechanics that all students should take. In the ME 840 course, the theoretical understanding gained in ME 830 is supplemented with material on numerical methods, discretization of equations, and stability constraints appropriate for developing and using computational methods of solution to fluid mechanics and convective heat transfer problems. Students augment these courses with additional courses in fluid mechanics and satisfy breadth requirements by selecting courses in the areas of Thermal Sciences, Mechanical and Dynamical Systems, and Solid and Structural Mechanics. Graduate Course and Research Topics (Profs. Benard, Brereton, Jaberi, Koochesfahani, Naguib) ExperimentalResearch Many fluid flow phenomena are too complicated to be understood fully or predicted accurately by either theory or computational methods. Sometimes the boundary conditions of real-world problems cannot be analyzed accurately. -

Architectural Engineering (Omaha) 1
Architectural Engineering (Omaha) 1 education in each of the three option areas and provides the professional ARCHITECTURAL practice topics that architectural engineers need later in their careers. ENGINEERING (OMAHA) The MAE year features a major interdisciplinary design project. The project requires the student to practice the design skills and understanding of building systems previously developed. Student Description teams complete a significant building design in a manner that closely Website: http://engineering.unl.edu/durhamschool/architectural- simulates professional practice. Industry and faculty members serve as engineering/ consultants to the students. Typically, students enter this design into the national Architectural Engineering Institute competition. Traditionally, our The architectural engineering (BSAE) undergraduate program is a students do very well at this competition. four-year program requiring 130 credit hours. A one-year master of architectural engineering (MAE) program of 36 credits is also offered. The Career Opportunities MAE degree is accredited by ABET, and almost all of our BSAE graduates Architectural engineering graduates normally enter the building design continue to complete the MAE degree. industry and become registered professional engineers. There are about 20 accredited architectural engineering programs in the country, so there Educational Objectives is a large unfulfilled demand for engineers educated in building design. In The following are the BSAE/MAE program educational objectives (PEOs): Nebraska, the home of several large architectural and engineering design firms, this is especially true. 1. Professional Accomplishment: The AE program will prepare graduates to become licensed professional engineers a few years Architectural engineering is accredited by the EAC-ABET, Inc. The after graduation. accreditation is attached to the one-year master of architectural 2. -

Mech 673 Energy Efficient Buildings with Good Indoor Air Quality 3 Cr
MECH 673 ENERGY EFFICIENT BUILDINGS WITH GOOD INDOOR AIR QUALITY 3 CR. The course covers energy consumption standards and codes in buildings; energy conservation measures in built in environment to enhance the building’s energy efficiency while maintaining space thermal comfort and indoor air quality requirement; fundamental ventilation, indoor-air-quality, infiltration natural and mechanical ventilation, importance and impact of indoor air quality on human health and energy performance of the building air conditioning system; and ASHRAE requirement for ventilation. Particular focus will be given to green energy alternative measures. An overview of the different heating, ventilation and air conditioning system designs is covered. Performance and energy consumption of the conventional air conditioning system (constant and variable air volume) as well as the hybrid integrated air conditioning systems will be discussed and compared. The course will include several demonstrations of concept experiments. Prerequisite: MECH 310. Technical Elective. Pre-requisite: MECH 310. Textbook: Handouts. References David Etheridge, 1996. Building Ventilation: Theory and Measurement. CRC Press. Ken Parsons. 1996. Human Thermal Environments: The Effects of Hot, Moderate, and Cold Environments on Human Health, Comfort and Performance. Second Edition, CRC Press. Hazim Awbi. 2003. Ventilation of Buildings, Spon Press; 2 edition. Yuanhui Zhang. 2004. Indoor Air Quality Engineering, CRC Press. Brigittta Nordquist, 2002. Ventilation and Window Opening in Schools. PhD thesis. Lund University, Sweden. Chad Dorgan, Robert Linder, and Charles Dorgan. 1999. Indoor air Quality Standards of Performance. American Society of Heating, Refrigerating, and Air-conditioning Engineers, Inc. Sustainable Building Technical Manual: Green Building Design, Construction and Operations, Public Technology, Inc., Washington, D.C., 1996. -

Air Compressors
Brown Hills College of Engineering & Technology Energy Conversion UNIT – 7 Air Compressors Working of a single stage reciprocating air compressor, Calculation of work input, Volumetric efficiency, Isothermal efficiency, Advantages of multi stage compression, Two stage compressor with- Inter-cooling, Perfect Inter cooling, Optimum intercooler pressure. Introduction : It is a mechanical component (machine) to compress the air with raise its pressure. The air compressor sucks air from the atmosphere and compresses it then further delivers with a high pressure to a storage vessel. From the storage vessel, it may be transmit by the channel (pipeline) to a place where the supply of compressed air is required. Afterward the compression of air requires some work to be done on it; therefore a compressor must be driven by some prime mover. Application of Air Compressor : It is used for operating pneumatic drills, riveters, road drills, paint spraying, in starting and supercharging of internal combustion engines, in gas turbine plants, jet engines and air motors, etc. It is also employ in the operation of lifts, rams, pumps etc. Classification of Air Compressors : 1. According to working : 2. According to action : 3. According to number of stages : • Reciprocating compressors • Single acting compressors • Single stage compressors • Rotary compressors • Double acting compressors • Multi-stage compressors Important Definitions : 1. Inlet pressure: It is the absolute pressure of air at the inlet of a compressor. 2. Discharge pressure: It is the absolute pressure of air at the outlet of a compressor. 3. Compression ratio (or pressure ratio): It is the ratio of discharge pressure to the inlet pressure. Since the discharge pressure is always more than the inlet pressure, therefore the value of compression ratio is more than unity. -

Mechanical Engineering Program Abet Course Syllabus
MECHANICAL ENGINEERING PROGRAM ABET COURSE SYLLABUS ME 302 Thermodynamics I (3 Units) Required Course Description: Properties of working fluids and fundamental relations for processes (2019-20 Catalog) involving the transfer of energy. First and second laws of thermodynamics, irreversibility and availability. 3 lectures. Prerequisite Courses: ME 212 and PHYS 132. Prerequisites by Topic: Engineering dynamics including work-energy theorem. Physics including an introduction to energy and energy transfer. Textbook: Fundamentals of Engineering Thermodynamics, 6th, 7th, or 8th (and/or other required Editions by Moran Shapiro, Boettner, and Bailey, John Wiley & material) Sons. References: None Course Coordinator/Instructor: Andrew Kean, Professor of ME Course Learning Outcomes: The student will be able to: 1. Logically define and analyze engineering problems involving work, heat transfer, and energy 2. Define a thermodynamic system, the concept of a thermodynamic state, and determination of properties for liquids, ideal and real gases, and fluids with phase changes 3. Synthesize these concepts through application of the first and second laws of thermodynamics to both closed and open systems 4. Evaluate of the limitations placed on processes and cycles by the laws of thermodynamics Relationship of Course to SO 1: Develop (D) Mechanical Engineering SO 2: Student Outcomes: SO 3: SO 4: SO 5: SO 6: SO 7: Topics Covered: Fundamental Concepts: thermodynamic system, surroundings, energy, work, heat transfer, power, units systems (3 lectures) Physical Properties: states, thermodynamic properties vs. non- properties, ideal gas, real gas, phase change, tabulated properties and their evaluation, graphical presentations. (7 lectures) First Law: energy can be neither produced nor destroyed, applied to closed and open systems, applied to processes and cycles. -

Experiment C2 Analog Thermostat Procedure
University of Notre Dame Aerospace and Mechanical Engineering AME 21216: Lab I Spring 2020 Experiment C2 Analog Thermostat Procedure Deliverables: Checked lab notebook, Technical Memo Overview A thermostat is a device that turns an actuator ON or OFF depending on the input from some sensor. Because of their abrupt switching, thermostats are often referred to as “bang-bang” controllers. The most common example is in an HVAC system, where the furnace or air conditioning is turned ON or OFF depending on the measured temperature in the room. In this lab, you will use a famous analog Op-Amp circuit known as a “Schmitt trigger” to switch a heater or a fan, depending on the temperature measured by a thermistor. The same circuit will also be used to switch a pressure relief valve depending on the measured pressure in a cylinder. The Schmitt trigger has a special feature known as hysteresis, where the threshold voltage changes depending on whether the output is ON or OFF. This hysteresis prevents the thermostat from slipping into rapid oscillation. Part I: System Identification and Characterization Background The resistive heater and thermistor used in this lab will be modeled as a lumped thermal mass. When the heater is off and the system is cooling, the thermal energy balance takes the form dT mcp = Ah(TAir − T ), (1) dt where T is the temperature of the system, TAir is the temperature of the ambient air, m is the system mass, cp is specific heat, A is the surface area, and h is the heat transfer coefficient. When the heater is on, the energy balance becomes dT mcp = Ah(TAir − T ) + q , (2) dt where q = iV is the heater power. -
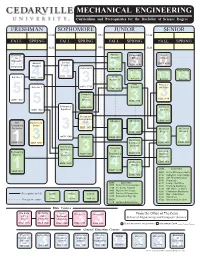
Mechanical Engineering
MECHANICAL ENGINEERING Curriculum and Prerequisites for the Bachelor of Science Degree FRESHMAN SOPHOMORE JUNIOR SENIOR CUM GPA CUM FALL SPRING FALL SPRING FALL SPRING FALL SPRING SEM GPA SEM Chemistry Thermo- Professional Senior for dynamics Ethics Seminar Engineers General General EGGN 4010 . EGGN 3110 Physics I Physics II H 3.5CHEM 1050 . Calculus III EGME 3110 3 0 5 Engineering Engineering Engineering PHYS 2120 Calculus I PHYS 2110 Fluid . 4 1-3Elective 1-3 4 Mechanics Elective 1-3Elective Heat 3 . Electrical Calculus II MATH 2710 Transfer EGME 3210 Machines 3. Computa- . 5 tional . EGEE 3530 MATH 1710 Methods 3 EGME 3150 . 3 5 Differential EGME 2050 Automatic MATH 1720 Equations 4 Controls Circuts and H Instrumenta- ME Labs I ME Labs II EGME 4660 The Digital Logic tion . 3 Engineering Design EGEE 2050 Profession ME Senior ME Senior 4 Design II EGME 3020 Design I 3 2. MATH 2740 Dynamics EGME2 3010 Kinematics EGGN 1110 EGCP3 1010 & Design of EGME 4810 EGME 4820 1 Machines 3 3 Statics and Properties Mechanics Engineering of Contemp. of EGME . 3610 Graphics EGME3 2630 Engineering Manufac. Materials 3 Materials Processes EGME 2430 EGME 2530 Mechanical 5 Design 3 EGME ELECTIVES EGME4 2410 EGME 1810 1 H 4050 Finite Difference Meth. EGME 3850 4160 Radiation Solar Energy 3 4210 Adv Fluid Mechanics 4250 Propulsion EGME ELECTIVES 4270 Comp. Fluid Flow 3050 Finite Element Analysis 4410 Fracture Mechanics 3130 Int. Comb. Engines 4530 Adv. Mech. of Mat’ls 3430 Physical Metallurgy 4550 Continuum Mechanics Prerequisite on left Credit Honors Course 3450 Plastics & Composites 4610 Dyn. -
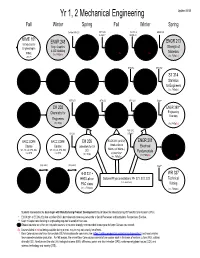
Mechanical Engineering Updated 05/20 Fall Winter Spring Fall Winter Spring
Yr 1, 2 Mechanical Engineering Updated 05/20 Fall Winter Spring Fall Winter Spring Co-Req MTH 251 MTH 252 PH 211 & ENGR 211 & Soph** ENGR 211 MIME 101 ENGR 248 ENGR 112 ENGR 213 Introduction to Introduction to ENGR 211** ENGR 212 Engr. Graphics Strength of Engineering in Engineering & 3-D Modeling Statics Dynamics (3cr, FWSpSu) (3cr, FWSpSu) Materials MIME (3cr, FWSpSu) Computing (3cr, F) (3cr, FWSpSu) (3cr, FWSpSu) MTH 254 MTH 252 MTH 251 MTH 252 MTH 254 MTH 256 ST 314 MTH 341 Differential Integral Vector Differential Statistics Linear Algebra Calculus Calculus Calculus Equations (3cr, FWSpSu) for Engineers (4cr, FWSpSu) (4cr, FWSpSu) (4cr, FWSpSu) (4cr, FWSpSu) (3cr, FWSpSu) MTH 251 MTH 252 MTH 254 Soph** CH 201 CH 202 PH 211 PH 212 PH 213 ENGR 390** Eng Chemistry for Chemistry for Physics Physics Physics ineering Economy Engineers Engineers w/Calculus w/Calculus w/Calculus (3cr, F,W) (3cr, W,Sp) (4cr, FWSpSu) (4cr, FWSpSu) (4cr, FWSpSu) (3cr, FWSpSu) MTH 252 CH 202 & Soph** BACC CORE BACC CORE CH 205 ECON 201 (or 202) ENGR 201 ENGR 202 Course † Course Laboratory for CH Introduction to Electrical Electrical WC, CD, LA, DPD, BIO, WC, CD, LA, DPD, BIO, 202 Micro- (or Macro-) Fundamentals Fund. II CGI, STS CGI, STS economics* (1cr, W,Sp) (3cr, FWSpSu) (3cr, WSpSu) (4cr, FWSpSu) (Any order) (Any order) Soph** WR 121 COMM HHS 231 + WR 327 English 111/114 HHS Lab or Optional Physics recitations: PH 221, 222, 223 Technical (1 cr. each term) Composition Speech PAC class Writing (3cr, FWSpSu) (3cr, FWSpSu) (2+1cr, FWSpSu) (3cr, FWSpSu) Students interested in the dual major with Manufacturing Product Development should follow the Manufacturing PD flowchart and Academic Plan. -

ME 312– Thermodynamics II (Required)
Department of Mechanical Engineering Mechanical Engineering Department ME 312– Thermodynamics II (Required) Catalog Description: ME 312 (3 0 3) A continuation of ME 311 including studies of irreversibility and combustion. Thermodynamic principles are applied to the analysis of power generation, refrigeration, and air-conditioning systems. Introduction to solar energy thermal processes, nuclear power plants, and direct energy conversion. Prerequisites: ME 311 - Thermodynamics I Reason for prerequisites: Thermodynamics II is the second part of a two-semester course on Thermodynamics. Textbook(s)/Materials Required: Yunus A. Cengel and Michael A. Boles. THERMODYNAMICS: An Engineering Approach , 4th Edition, McGraw-Hill, NY, 2002, ISBN 0-07-238332-1 Course Supervisor: Dr. Boris Khusid Pre-requisite by topic: 1. Ideal gases 2. Thermodynamic properties of pure substances 3. First law analysis of open systems, steady and transient processes 4. The second law of thermodynamics and entropy 5. Entropy change during processes 6. Energy, work potential Course Objectives1: 1. To develop the student’s ability to apply the principles of thermodynamics to the optimal design of the basic energy conversion systems: power generation, refrigeration, air-conditioning, and combustion. (A, B, C, D) 2. To develop the student’s ability to use thermodynamic relations and the property tables and charts for the analysis of energy conversion systems in the course of their operation. (A, B, C, D) 3. To develop the student’s ability to apply the first and the second laws of thermodynamics to the optimization of the basic energy conversion systems. (A, B, C, D) 4. To provide the students with some knowledge and analysis skills associated with the principles of operation and applications of the main energy conversion systems. -

Engineering Thermodynamics
Department of Mechanical Engineering Indian Institute of Technology New Delhi I Semester -- 2014 – 2015 MCL 140 ENGINEERING THERMODYNAMICS Course Contents • Introduction Role of Thermodynamics in Engineering and Science -- Applications of Thermodynamics : Power Generation, Thermal Environment Control, Cooling of Electrical Systems and Electronic Devices, Analysis of Manufacturing Processes, Study of Biological Systems, Pollution Control - -Founders of Thermodynamics. • Basic Definitions and Units The Thermodynamic System and The Control Volume -- Surroundings -- Concept of Universe - - Macroscopic and Microscopic Analysis -- Definition of Substance -- Properties of Substance : Intensive and Extensive -- Mathematical Representation of Property -- State of substance -- Thermodynamic Equilibrium -- Concept of Quasi-- Equilibrium -- Process and Cycle -- Fundamental Units -- Units of Force, Energy, Specific Volume, Pressure etc. -- Equality of Temperature -- The Zeroeth Law of Thermodynamics -- Temperature Scales -- The International Scale of 1990. • The Pure Substance Definition of Pure Substance -- Facts about Pure Substances -- Vapor -- liquid -- solid Phase Equilibrium -- Equation of State for the Vapor Phase : Simple substance, Ideal Gase Characterization, Ideal Gas Equation,Compressibility Effects and Resulting Equations of State -- Real Gases. • Heat and Work Definition of Thermodynamic Work -- Units for Work -- Forms of Work – Definition of Heat -- Inter Convertibility of Heat/work into Work/heat -- Governing Principles -- Sign Convention. • First Law of Thermodynamics Statement of First Law of Thermodynamics : First Law for Cyclic Process, First Law for Change of State of A System : Internal Energy, A New Thermodynamic Property -- Enthalpy -- The Constant Volume and Constant Pressure Specific Heats -- The internal Energy, Enthalpy and Specific Heats of An Ideal Gas – First Law as a Rate Equation -- First Law Applied to a Control Volume -- The SSSF and USUF Processes. -

Industry Booklet.Pub
Dr. Ardalan Vahidi discussing research with three Ph.D. students inside the EMC2 (Efficient Mobility via Connectivity and Control) lab. MECHANICAL ENGINEERING Using cutting edge research on autonomous driving, vehicle- infrastructure communication, and hybrid electric vehicle technologies, researchers are able to lower energy consumption and improve transportation safety. Clemson University’s Department of Mechanical Engineering is now accepting applications for its Master of Science (M.S.) and Doctor of Philosophy (Ph.D.) degrees. Classes are offered every semester. Designed for Working Charleston on a rotating basis to engineering. Subject areas covered Professionals ensure students on both campuses include experimental, analytical and have in-person contact. Recorded computational work ranging from The ME graduate program is lectures are available and cloud manufacturing systems to material designed for working engineers, with archived for 24/7 retrieval, allowing processing; mechanics of materials classes generally held online and in- students to make progress and view to thermodynamics; dynamic person at the Zucker Family class lectures regardless of job or systems and controls to engineering Graduate Education Center in North personal obligations. Faculty hold design practice and methods. Charleston during the late scheduled office hours in order to be Students can elect to engage in a afternoons and evenings. Students available to meet with student research-oriented graduate degree or are not be required to travel to virtually and one-on-one in North enroll in a coursework only option Clemson’s main campus. Charleston. with no thesis required. Both degrees are identical in Courses are offered each semester requirements and expectations to in a traditional face-to-face The Program the programs currently offered on classroom format and in “real time” Graduate degrees in mechanical Clemson’s main campus.