And Coumarin-1-Ylmehtyl Photoremovable Protecting Groups
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydroxylamine-O -Sulfonic Acid — a Versatile Synthetic Reagent
Hydroxylamine-O -sulfonic acid — a versatile synthetic reagent Raymond G. Wallacef School of Chemistry Brunei University Uxbridge, Middlesex UBS 3PH Great Britain imidazoli nones and related derivatives are time to these various modes of reaction. discussed in the review. Many of these The uses of HOSA as a reagent are organiz preparations can be carried out in high ed below according to the different syn yield, thetic transformations that it can bring about. Hydroxylamine-Osulfonic acid, NHj-OSOjH (abbreviated to HOSA in Probably by far the most well known this article) has become in recent years and explored reactions of HOSA are commercially available. Although much animation reactions, illustrating elec fruitful chemistry has been carried out us trophilic attack by HOSA, with amination ing HOSA, to this author's knowledge, on nitrogen being the most important, there has been no systematic review in although a significant number of English* of its use as a synthetic reagent. It animations on both carbon and sulfur have is a chemically interesting compound been reported, Amination on phosphorus because of the ability of the nitrogen center also occurs. to act in the role of both nucleophile and AMINATION electrophile, dependent on circumstances, Synopsis (a) At a nitrogen atom and thus it has proved to be a reagent of Hydroxylamine-0-sulfonic acid (0 Preparation of mono- and di- great synthetic versatility. (HOSA) has only recently become widely substituted hydrazines and trisubstituied commercially available despite the fact that H,N-Nu hydrazinium salts it has proved to be a valuable synthetic reagent in preparative organic chemistry. -

And TMS Ethers to Sulfonate Esters Using Fecl3-Montmorillonite K-10 Clay Barahman Movassagha and Salman Shokri Department of Chemistry, K
An Efficient One-Pot Conversion of THP- and TMS Ethers to Sulfonate Esters Using FeCl3-Montmorillonite K-10 Clay Barahman Movassagha and Salman Shokri Department of Chemistry, K. N. Toosi University of Technology, P. O. Box 16315-1618, Tehran, Iran a Kermanshah Oil Refining Company, Kermanshah, Iran Reprint requests to Dr. B. Movassagh. E-mail: [email protected] Z. Naturforsch. 60b, 763 – 765 (2005); received March 29, 2005 Various tetrahydropyranyl and trimethylsilyl ethers are efficiently transformed into the correspond- ing sulfonate esters with sulfonyl chlorides in the presence of FeCl3-Montmorillonite K-10 clay. Key words: Sulfonate Esters, FeCl3-Montmorillonite K-10, Trimethylsilyl Ethers, Tetrahydropyranyl Ethers, Clay Catalyst Sulfonate esters are especially useful substrates in sions of THP and TMS ethers into the correspond- nucleophilic substitution reactions used in synthesis. ing carboxylic esters in the presence of Montmoril- Their preparation from alcohols is an effective way lonite K-10 clay [7, 8]. In continuation of our ongo- of installing a reactive leaving group, e.g. on an alkyl ing program to develop environmentally benign meth- chain. p-Toluenesulfonate- and methanesulfonate es- ods using supported reagents, this article describes ters are the most frequently used groups for preparative a new, simple, and efficient one-pot protocol for the work. The usual method for preparation of sulfonates deprotection-sulfonylation of THP and TMS ethers relies on the use of the corresponding sulfonyl chlo- with sulfonyl chlorides in refluxing acetonitrile using ride or anhydride in the presence of triethylamine [1], FeCl3 impregnated Montmorillonite K-10 as a mild pyridine [2], 1,4-diazabicyclo[2.2.2]octane (DABCO) and non-corrosive solid catalyst (Scheme 1). -

Studies Toward the Synthesis of Photolabile HNO Donors–An
STUDIES TOWARD THE SYNTHESIS OF PHOTOLABILE HNO DONORS – AN EXPLORATION OF SELECTIVITY FOR HNO GENERATION A thesis submitted to the Kent State University Honors College in partial fulfillment of the requirements for Departmental Honors by Zachary Alan Fejedelem August, 2015 Thesis written by Zachary Alan Fejedelem Approved by ________________________________________________________________, Advisor ________________________________________________________________, Advisor ________________________________________________________________, Chair, Department of Chemistry & Biochemistry Accepted by _____________________________________________________, Dean, Honors College ii TABLE OF CONTENTS LIST OF FIGURES……………………………………………………...…………….…vi LIST OF TABLES………………………………………………………………………..ix ACKNOWLEDGMENTS………………………………………………….......................x CHAPTER 1. INTRODUCTION……………………………………………………..….1 1.1 Introduction to HNO……………………………………………....1 1.1.1 The unique qualities of HNO……………………………………...1 1.1.2 Drawbacks and issues of HNO…………………………………....5 1.1.3 Importance of HNO donors……………………………….………6 1.2 Examples of HNO donors………………………………………...7 1.2.1 Angeli’s Salt (AS)………………………………………………...7 1.2.2 Piloty’s Acid (PA)………………………………………………...8 1.2.3 HNO-generating diazeniumdiolates…………………………..…12 1.2.4 Cyanamide…………………………………………………….…12 1.2.5 Nitrosocarbonyls…………………………………………………14 1.2.6 Hydroxylamine…………………………………………………..16 1.2.7 α-Acyloxy-C-nitroso compounds………………………………..16 1.2.8 Photolabile HNO and NO donors………………………………..17 1.3 Our research group’s family of current HNO donors…………....20 iii 1.3.1 Previous work on first family of photoactivatable HNO donors..20 1.3.2 Previous synthesis of target photolabile HNO donors…………..22 1.4 Photolysis results from HNO donors 22………………………....25 1.4.1 Photolysis results of first HNO donor 22a……………………....25 1.4.2 Proposed photolysis mechanism………………………………...27 1.5 Thesis project goal……………………………………………....29 1.5.1 Thesis project……………………………………………………29 1.5.2 Proposed photolysis mechanism………………………………...31 2. -

A Method for the Production of Sulfate Or Sulfonate Esters
(19) *EP002851362B1* (11) EP 2 851 362 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 303/24 (2006.01) C07C 303/28 (2006.01) (2006.01) (2006.01) 27.11.2019 Bulletin 2019/48 C07C 305/06 C07C 305/08 C07C 305/20 (2006.01) C07C 305/24 (2006.01) (2006.01) (21) Application number: 13185032.3 C07C 309/73 (22) Date of filing: 18.09.2013 (54) A method for the production of sulfate or sulfonate esters Verfahren zur Herstellung von Sulfat oder Sulfonatestern Procédé pour la production d’esters de sulfate ou de sulfonate (84) Designated Contracting States: (56) References cited: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • DENIZ GUNES ET AL: "ALIPHATIC THIOETHERS GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO BY S-ALKYLATION OF THIOLS VIA TRIALKYL PL PT RO RS SE SI SK SM TR BORATES", PHOSPHORUS, SULFUR AND SILICON AND THE RELATED ELEMENTS, (43) Date of publication of application: TAYLOR & FRANCIS INC, US, vol. 185, no. 8, 1 25.03.2015 Bulletin 2015/13 January 2010 (2010-01-01), pages 1685-1690, XP008165903, ISSN: 1042-6507, DOI: (73) Proprietor: Ulusal Bor Arastirma Enstitusu 10.1080/10426500903213563 [retrieved on 06520 Ankara (TR) 2010-08-02] • OKI ET AL: "Solvothermal synthesis of carbon (72) Inventors: nanotube-B2O3 nanocomposite using tributyl • Bicak, Niyazi borate as boron oxide source", INORGANIC 34469 Istanbul (TR) CHEMISTRY COMMUNICATIONS, ELSEVIER, • Gunes, Deniz AMSTERDAM, NL, vol. -

Fundamental Studies on 2,4,6- Trichlorophenyl Sulfonate Esters
University College London Fundamental Studies on 2,4,6- Trichlorophenyl Sulfonate Esters By Lynsey J. Geldeard Doctor of Philosophy Faculty of Mathematical and Physical Sciences Department of Chemistry Declaration The work described in this thesis is the work of the author and has not previously been submitted to this or any other university for any other degree. Lynsey Geldeard February 2009 Abstract This thesis describes the application of 2,4,6-trichlorophenylsulfonate esters in the synthesis of sulfonamides. The sulfonamide unit is an important structural motif due to its frequent occurrence in a range of pharmaceuticals, particularly antibiotics. Sulfonamides can be readily synthesised from pentafluorophenyl (PFP) sulfonate esters and as an expansion to this 2,4,6 trichlorophenyl (TCP) sulfonates have been developed. These have the added advantage of lower toxicity and reduced cost of trichlorophenol. TCP sulfonates can be synthesised directly from sulfonic acids via activation by triphenylphosphine ditriflate in moderate to excellent yields. These compounds can then be utilised in the synthesis of sulfonamides and suitable conditions for reactions with both simple aliphatic amines and more challenging anilines have been found. The differing reactivity’s of the TCP and PFP sulfonate esters have been exploited in selective sulfonamide formation. The greater stability of TCP sulfonate in comparison to PFP sulfonate also means that a broader range of transformations can be achieved in its presence. This has been shown particularly in the application of palladium chemistry to synthesise more elaborate TCP sulfonates. Also, the synthesis of novel amino acids have been targeted inorder to further demonstrate the stability of the group when performing more diverse reactions on remote sites in the molecule. -

Fabrication and Use of Organic Electrochemical Transistors for Sensing of Metabolites in Aqueous Media
applied sciences Review Fabrication and Use of Organic Electrochemical Transistors for Sensing of Metabolites in Aqueous Media Benoît Piro 1,*, Giorgio Mattana 1, Samia Zrig 1, Guillaume Anquetin 1, Nicolas Battaglini 1, Dany Capitao 2, Antoine Maurin 2 and Steeve Reisberg 1 1 Université Paris Diderot, Sorbonne Paris Cité, ITODYS, UMR 7086 CNRS, 15 rue J-A de Baïf, 75205 Paris CEDEX 13, France; [email protected] (G.M.); [email protected] (S.Z.); [email protected] (G.A.); [email protected] (N.B.); [email protected] (S.R.) 2 ValoTec, 1 Mail du Professeur Georges Mathé, 94800 Villejuif, France; [email protected] (D.C.); [email protected] (A.M.) * Correspondence: [email protected]; Tel.: +33-(0)1-5727-7224 Received: 9 May 2018; Accepted: 1 June 2018; Published: 4 June 2018 Featured Application: Sensing organic molecules in water. Abstract: This review first recalls the basic functioning principles of organic electrochemical transistors (OECTs) then focuses on the transduction mechanisms applicable to OECTs. Materials constituting the active semiconducting part are reviewed, from the historical conducting polymers (polyaniline, polypyrrole) to the actual gold standard, poly-3,4-ethylenedioxythiophene: polystyrene sulfonic acid (PEDOT:PSS), as well as the methods used to fabricate these transistors. The review then focuses on applications of OECTs for the detection of small molecules and more particularly of metabolites, with a distinction between enzymatic and non-enzymatic transduction pathways. Finally, the few patents registered on the topic of OECT-based biosensors are reviewed, and new tracks of improvement are proposed. -

Oxime Sulfonate Chemistry for Advanced Microlithography
Journal of Photopolymer Science and Technology Volume 20, Number 5 (2007) 637-642 @TAPJ Oxime Sulfonate Chemistry for Advanced Microlithography Hitoshi Yamato, Toshikage Asakura, Yuichi Nishimae, Akira Matsumoto, Junichi Tanabe, Jean-Luc Birbaum, Peter Murer, Tobias Hintermann and Masaki Ohwa Technology Center Electronic Materials, Coating Effects Segment, Ciba Specialty Chemicals K.K., 7-1-13, Doi-cho, Amagasaki, Japan 660-0083 Oxime sulfonate compounds are one of the important chemistry as photoacid generator for advanced lithography application and practically used in mass production of semiconductor chips. This chemistry is adjustable for various applications like g-/h-/i-line, KrF and ArF lithography. This paper describes an overview of oxime sulfonate chemistry for semiconductor resist application, including commercially available products. Keywords: oxime sulfonate, photoacid generator, g-line, i-line, KrF, ArF, chemically amplified resist 1. Introduction The development of semiconductor chips is desired resist performance such as resolution, making progress with an astonishing speed, depth-of-focus, line edge roughness and etching enabling electronic apparatus smaller, higher resistance, the acid-labile polymers have been speed, and higher performance. This dynamic intensively studied first and developed notably. For advancement is significantly attributable from the further improvement of the CA resist, PAG is the development of photolithography technology. At key ingredient. Several chemistries have been an early era of integrated circuits (IC) extensively investigated as the photoactive manufacturing lithography technique was component for the CA resists, e.g., iodonium introduced with light source of g-line wavelength sulfonate, sulfonium sulfonate, (436 nm). For further miniaturization of IC the diazomethanedisulfone, N-sulfonyloxyimido wavelength of light source is getting shorter and compounds, nitrobenzyl sulfonate, and so on [3]. -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Functional Group Interconversions 119
FUNCTIONAL GROUP INTERCONVERSIONS 119 Functional Group Interconversions C&S Chapter 3 #1; 2; 4a,b, e; 5a, b, d; 6a,b,c,d; 8 1 sulfonates 2 halides 3 nitriles 4 azides 5 amines 6 esters and lactones 7 amides and lactams Sulfonate Esters - reaction of an alcohols (1° or 2°) with a sulfonyl chloride O R'= R'SO2Cl CH3 mesylate R OH R O S R' triflate O CF3 sulfonate ester CH3 tosylate - sulfonate esters are very good leaving groups. Elimination is often a competing side reaction Halides - halides are good leaving groups with the order of reactivity in SN2 reactions being I>Br>Cl. Halides are less reactive than sulfonate esters, however elimination as a competing side reaction is also reduced. - sulfonate esters can be converted to halides with the sodium halide in acetone at reflux. Chlorides are also converted to either bromides or iodides in the same fashion (Finkelstein Reaction). O X- R O S R' R X X= Cl, Br, I O NaI, acetone reflux R Cl R I - conversion of hydroxyl groups to halides: Organic Reactions 1983, 29, 1 R OH R X - R-OH to R-Cl - SOCl2 - Ph3P, CCl4 - Ph3P, Cl2 - Ph3P, Cl3CCOCCl3 FUNCTIONAL GROUP INTERCONVERSIONS 120 - R-OH to R-Br - PBr3, pyridine - Ph3P, CBr4 - Ph3P, Br2 - R-OH to R-I - Ph3P, DEAD, MeI Nitriles - displacement of halides or sulfonates with cyanide anion KCN, 18-C-6 DMSO R X R C N - dehydration of amides O R C N R NH2 - POCl3, pyridine - TsCl, pyridine - P2O5 - SOCl2 - Reaction of esters and lactones with dimethylaluminium amide TL 1979, 4907 Me H3C Me2AlNH2 OH JOC 1987, 52, 1309 O NC Ar O Ar - Dehydration of oximes OH N H2NOH•HCl P2O5 R CHO R C N R H - Oxidation of hydrazones O O N C Tetrahedron Lett. -
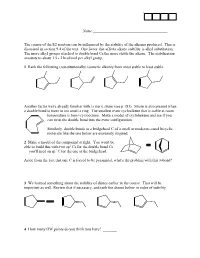
The Course of the E2 Reaction Can Be Influenced by the Stability of the Alkenes Produced
Name ______________________________________ The course of the E2 reaction can be influenced by the stability of the alkenes produced. This is discussed in section 9.4 of the text. One factor that affects alkene stability is alkyl substitution. The more alkyl groups attached to double bond Cs the more stable the alkene. The stabilization amounts to about 1.5 - 2 kcal/mol per alkyl group. 1 Rank the following (constitutionally) isomeric alkenes from most stable to least stable. Another factor we're already familiar with is steric strain (see p 181). Strain is also present when a double bond is trans in too small a ring. The smallest trans cycloalkene that is stable at room temperature is trans-cyclooctene. Make a model of cyclohexene and see if you can twist the double bond into the trans configuration. Similarly, double bonds to a bridgehead C of a small or moderate-sized bicyclic molecule like the one below are extremely strained. 2 Make a model of the compound at right. You won't be able to build this with two sp2 Cs for the double bond Cs — you'll need an sp3 C for the one at the bridgehead. Aside from the fact that one C is forced to be pyramidal, what's the problem with this π-bond? 3 We learned something about the stability of dienes earlier in the course. That will be important as well. Review that if necessary, and rank the dienes below in order of stability. 4 How many HW points do you think you have? _______ Lecture outline E2 reaction — Elimination, bimolecular. -

Sulfonate Esters – How Real Is the Risk?
Sulfonate Esters – How Real is the Risk? Summary of Key Findings from PQRI Studies of the Reaction Between Sulfonic acids and Alcohols A Teasdale Introduction – Description of the issue There have been growing concerns expressed by regulators in relation to the potential generation of genotoxic impurities as a result of interactions between strong acids and alcohols. This has centred primarily on sulfonate esters, theoretical impurities resulting from interaction between sulfonic acids and alcohols. O O R S OH R' OH + R S OR' + OH2 O O R - alkyl / aryl R'- alkyl - methyl / ethyl / isopropyl etc. Global Process R&D Process Global Introduction – Description of the Issue Issue was discussed at length at the DIA meeting on genotoxic impurities – November 2005 – FDA expressed significant concerns over use of sulfonic acids as counterions. It was clear at the meeting that many companies had carried out in house studies – Showing some understanding of levels formed and how to control them. Clear challenge from this meeting going forward was for industry to build on these studies – To provide clear evidence of this understanding and to publish. – PQRI Initiative borne out of this need. Global Process R&D Process Global What is PQRI (www.pqri.org)? The Product Quality Research Institute www.pqri.org “… (PQRI) is a collaborative process involving FDA’s Center for Drug Evaluation and Research (CDER), Industry, and Academia.” “The mission of PQRI is to conduct research to generate specific scientific information that should be submitted in a regulatory filing to CDER.” “…PQRI tackles projects to ensure the quality, safety and performance of drug products.” Global Process R&D Process Global Study Model Key aspect of the work was independent verification of data. -

Linear Alkylbenzene Sulfonate (Las)
OECD SIDS LINEAR ALKYLBENZENE SULFONATE (LAS) FOREWORD INTRODUCITON LINEAR ALKYLBENZENE SULFONATE (LAS) 1322-98-1 Decylbenzene sulfonic acid, sodium salt 25155-30-0 Dodecylbenzene sulfonic acid, sodium salt 26248-24-8 Tridecylbenzene sulfonic acid, sodium salt 27636-75-5 Undecylbenzene sulfonic acid, sodium salt 68081-81-2 C10-16 Monoalkylbenzene sulfonic acid, sodium salt 68411-30-3 C10-13 Alkylbenzene sulfonic acid, sodium salt 69669-44-9 C10-14 Alkyl deriv benzene sulfonic acid, sodium salt 85117-50-6 C10-14 Monoalkylbenzene sulfonic acid, sodium salt 90194-45-9 C10-13 Alkyl deriv benzene sulfonic acid, sodium salt 127184-52-5 4-C 10-13-sec Alkyl deriv benzene sulfonic acid, sodium salt UNEP PUBLICATIONS 1 OECD SIDS LINEAR ALKYLBENZENE SULFONATE (LAS) SIDS INITIAL ASSESSMENT REPORT For 20th SIAM Paris, France, 19-21 April, 2005 1. Chemical Name: Linear Alkylbenzene Sulfonate (LAS) 2. CAS Numbers: 1322-98-1 Decylbenzene sulfonic acid, sodium salt 25155-30-0 Dodecylbenzene sulfonic acid, sodium salt 26248-24-8 Tridecylbenzene sulfonic acid, sodium salt 27636-75-5 Undecylbenzene sulfonic acid, sodium salt 68081-81-2 C10-16 Monoalkylbenzene sulfonic acid, sodium salt 68411-30-3 C10-13 Alkylbenzene sulfonic acid, sodium salt 69669-44-9 C10-14 Alkyl deriv benzene sulfonic acid, sodium salt 85117-50-6 C10-14 Monoalkylbenzene sulfonic acid, sodium salt 90194-45-9 C10-13 Alkyl deriv benzene sulfonic acid, sodium salt 127184-52-5 4-C10-13-sec Alkyl deriv benzene sulfonic acid, sodium salt 3. Sponsor Country: United States National SIDS Contact Point in Sponsor Country: Oscar Hernandez, Director U.S.