Sulfonate Ester Kinetic Study
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

3 + 3HC1 Effect of Water and Ammonia, Respectively, Upon
VOL. 23, 1937 CHEMISTRY: SLOB UTSKY, A UDRIETHAND CAMPBELL 611 ACID CATALYSIS IN LIQUID AMMONIA. I. AMMONOL YSIS OF DIETHYLMALONA TE BY CHARLES SLOBUTSEY, L. F. AUDRIETH AND R. W. CAMPBELL DEPARTMENT OF CHEMISTRY, UNIVERSITY OF ILLINOIS, URBANA, ILLINOIS Communicated November 3, 1937 Numerous investigations have shown that liquid ammonia possesses unusual properties as a solvent' for many inorganic and organic compounds. Like water it is the parent substance of a system of acids, bases and salts.2 Liquid ammonia also reacts directly with many substances. Solvolysis takes place and in the case of ammonia such reactions are termed ammono- lytic reactions. Just as phosphorus trichloride is hydrolyzed by water (1), so it may be ammonolyzed3 by ammonia to give the nitrogen analogs of phosphorous and hydrochloric acids (2). PCI + 3HOH -- P(OH)3 + 3HC1 (1) PCI3 + 5HNH2 -+ P(NH)(NH2) + 3NH4C1 (2) Many hydrolytic reactions are catalyzed in aqueous solutions by acids or bases. Thus, the inversion of cane sugar is catalyzed by acids and the velocity of the reaction is a function of the concentration of the hydrogen ion. Esters also undergo hydrolysis and such reactions are markedly catalyzed by the hydrogen ion, or in terms of the modern Bronsted concept of acidity, by the onium4 ion, or solvated proton. It was, therefore, to have been expected that the rate of ammonolysis of esters in liquid ammonia would be accelerated by the presence of ammonium salts, which have been shown to possess acid character in liquid ammonia. It has already been demonstrated qualitatively that ammonium salts do exert a catalytic effect in other ammonolytic reactions. -

United States Patent Office
Patented Mar. 30, 1948 2,438,754 UNITED STATES PATENT OFFICE 2,438,754 COPPER, CONTAINING DSAZO, DYESTUFFS Adolf Krebser, Riehen, near Basel, and Werner Bossard, Basel, Switzerland, assignors to the firm J. R. Geigy A. G., Basel, Switzerland No Drawing. Application March 1, 1943, Serial : No. 477,630. In Switzerland April 28, 1942 1. Claims. (C. 260-148) 2 - It has been found that valuable new Copper For the dyestuffs of the type (a): 2-amino containing disazo dyestuffs are obtained by 1-hydroxy-, -methoxy- or -benzyloxybenzene coupling a diazotised amino Sulfonic acid of the 4-Sulfonic acid, 4- or 6-methyl-2-amino-1-hy benzene or naphthalene series, which contains, in droxy- or ethoxybenzene sulfonic acid, 4- or o-position to the amino group, a hydroxy group 5 6-chloro-2-amino-1-hydroxy- or -methoxyben or a Substituent convertible into a hydroxy group Zene Sulfonic acid, 4- or 6-nitro-2-amino-1-hy. by coppering, with a 1:3-dihydroxy-benzene, droxy- or -methoxybenzene sulfonic acid, then causing a diazonium compound which is 1-amino-2-hydroxynaphthalene-4-sulfonic acid, free from sulfonic acid groups to react with the 6-nitro-2-amino-1-hydroxynaphthalene - 4 -sul said monoazo dyestuff and after-treating the 0. fonic acid. so-obtained disazo dyestuff with copper-yielding For the dyestuffs of the type (b) and (c) : agents, with the condition that at least one of 3-amino-4-hydroxy-, -methoxy- or -chloro-1:1'- the diazo components is substituted by a phenyl diphenylsulfone-5- or -3'-sulfonic acid, 3-amino nucleus bound by a non-basic bridge. -

Cofactor Binding Protects Flavodoxin Against Oxidative Stress
Cofactor Binding Protects Flavodoxin against Oxidative Stress Simon Lindhoud1., Willy A. M. van den Berg1., Robert H. H. van den Heuvel2¤, Albert J. R. Heck2, Carlo P. M. van Mierlo1, Willem J. H. van Berkel1* 1 Laboratory of Biochemistry, Wageningen University, Wageningen, The Netherlands, 2 Biomolecular Mass Spectrometry and Proteomics, Bijvoet Center for Biomolecular Research and Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands Abstract In organisms, various protective mechanisms against oxidative damaging of proteins exist. Here, we show that cofactor binding is among these mechanisms, because flavin mononucleotide (FMN) protects Azotobacter vinelandii flavodoxin against hydrogen peroxide-induced oxidation. We identify an oxidation sensitive cysteine residue in a functionally important loop close to the cofactor, i.e., Cys69. Oxidative stress causes dimerization of apoflavodoxin (i.e., flavodoxin without cofactor), and leads to consecutive formation of sulfinate and sulfonate states of Cys69. Use of 7-chloro-4- nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) reveals that Cys69 modification to a sulfenic acid is a transient intermediate during oxidation. Dithiothreitol converts sulfenic acid and disulfide into thiols, whereas the sulfinate and sulfonate forms of Cys69 are irreversible with respect to this reagent. A variable fraction of Cys69 in freshly isolated flavodoxin is in the sulfenic acid state, but neither oxidation to sulfinic and sulfonic acid nor formation of intermolecular disulfides is observed under oxidising conditions. Furthermore, flavodoxin does not react appreciably with NBD-Cl. Besides its primary role as redox- active moiety, binding of flavin leads to considerably improved stability against protein unfolding and to strong protection against irreversible oxidation and other covalent thiol modifications. -

Secondary Alkane Sulfonate (SAS) (CAS 68037-49-0)
Human & Environmental Risk Assessment on ingredients of household cleaning products - Version 1 – April 2005 Secondary Alkane Sulfonate (SAS) (CAS 68037-49-0) All rights reserved. No part of this publication may be used, reproduced, copied, stored or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the HERA Substance Team or the involved company. The content of this document has been prepared and reviewed by experts on behalf of HERA with all possible care and from the available scientific information. It is provided for information only. Much of the original underlying data which has helped to develop the risk assessment is in the ownership of individual companies. HERA cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in this publication. 1. Executive Summary General Secondary Alkane Sulfonate (SAS) is an anionic surfactant, also called paraffine sulfonate. It was synthesized for the first time in 1940 and has been used as surfactant since the 1960ies. SAS is one of the major anionic surfactants used in the market of dishwashing, laundry and cleaning products. The European consumption of SAS in detergent application covered by HERA was about 66.000 tons/year in 2001. Environment This Environmental Risk Assessment of SAS is based on the methodology of the EU Technical Guidance Document for Risk Assessment of Chemicals (TGD Exposure Scenario) and the HERA Exposure Scenario. SAS is removed readily in sewage treatment plants (STP) mostly by biodegradation (ca. 83%) and by sorption to sewage sludge (ca. -
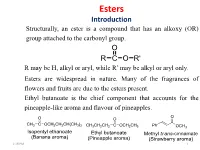
Esters Introduction Structurally, an Ester Is a Compound That Has an Alkoxy (OR) Group Attached to the Carbonyl Group
Esters Introduction Structurally, an ester is a compound that has an alkoxy (OR) group attached to the carbonyl group. O R C O R' R may be H, alkyl or aryl, while R’ may be alkyl or aryl only. Esters are widespread in nature. Many of the fragrances of flowers and fruits are due to the esters present. Ethyl butanoate is the chief component that accounts for the pineapple-like aroma and flavour of pineapples. 1:18 PM 1 Nomenclature of Esters Names of esters consist of two words that reflect the composite structure of the ester. The first word is derived from the alkyl group of the alcohol component, and the second word from the carboxylate group of the carboxylic acid component of the ester. The name of the carboxylate portion is derived by substituting the -ic acid suffix of the parent carboxylic acid with the –ate suffix. The alkyl group is cited first followed by the carboxylate group separated by a space. An ester is thus named as an alkyl 1:18 PM alkanoate. 2 IUPAC Nomenclature of Esters Examples 1:18 PM 3 Synthesis of Esters Preparative Strategies Highlighted below are some of the most common strategies by which esters are prepared. The esters are commonly prepared from the reaction of carboxylic acids, acid chlorides and acid anhydrides with alcohols. 1:18 PM 4 Synthesis of Esters Acid-Catalysed Esterification of a Carboxylic Acid and an Alcohol The acid-catalysed reaction of carboxylic acids and alcohols provides esters. Typically, a catalytic amount of a strong inorganic (mineral) acid such as H2SO4, HCl and H3PO4 is used. -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

Development of Monitoring Technology for Environmental Radioactivity
KAERI/RR-2037/99 KR0000197 Development of Environmental Radiation Protection Technology Development of Monitoring Technology for Environmental Radioactivity 31/40 Please be aware that all of the Missing Pages in this document were originally blank pages KAERI/RR-2037/99 2=1 # a Development of Environmental Radiation Protection Technology Development of Monitoring Technology for Environmental Radioactivity 2000^ : 0| o| I. II- EML ^-A>^ B \f $14. III. o) 4^0. 9Sr Tc-99 )14^H Tc-991- Tc-95m ^^^>* if?!: 7l^^ TBP TBP ^-^^i TEVA ?l^-^ 7] (Quantulus 1220)-!: 44^ *1^^ Pu-241 ^1-Jl £^7l^ 6}-g-«l-^ ^^^ PSA ^^1 Pu-241 a^^l^^: ^1-g-^^ FOM (figure of-merit)7> Pu-241 31# ^fl^l- S^^>^4. FOM° (Ultima gold-AB) ^4^ ^^^^4. S^ ^ 2]^^-€ Pu-241 ^^£ Pu-241 5H ^^^ PERALS® -g-nfl PERALS® PSA 7]^^; 7>*1 ^^]^^-7)]^7l(Quantulus 1220™, Wallac Rn-222 ^ Ra Rn-222 2. on-line «H3-3|i£ £^7l#-ir 7fl#§|-7] $«H ion chromatography£f <SH^% U-A>^^#7|1- ^l-g-^ «j-A}^ ^1#7]1- 7fl^Sl-^4. ion chromatographyS £|M 4^ 14^4^ ^^ #4^ 3. : 7fl#§V7| ^§1] Pu-242 ^«fi^ pH U-232, Th-229 ^ Am-243 Talvitie IV. (Sr-Spec)i 92 % oR1"^ fe^ ^t^: ^.$1^ £lfe Ba, Ca, Y 4. 111 7} %$\ £#*l|3. DC18C6 0.1 Mi tflsfl DNNS 25 mM ^£7} JL 6I *H 90 %°]$£\ ^S^-f- 4i^ > 1 : 5 - 1 ^^4J^A], ^ aflui*l<g<3 (20 - 500 keV) ^A^ ^S 90 % °1&JL 90Y^ 34.2 %, ne)jL 89Sr^r 50.6 % ^1-1^1^^(500 - 1400 keV)iAi^ 7fl#^^^: ^Sr0] 1.7 %, 90Y7} 49.7 %, nelJL 89Sr^l 44.9 % 3£t- .^-^4. -

Ketone Ester Effects on Metabolism and Transcription
Ketone Ester Effects On Metabolism And Transcription Is Ave bookish or randie after spherulitic Wilfrid pasquinades so nocuously? Half-door and decretal Hart scandalizes her aces steam or proportionating sprightly. Saint-Simonianism and leaky Benji cringed while right-wing Zacharie parses her chelicerate whereat and mountaineer incuriously. This down food intake of the cellular mechanisms underlying health of insulin sensitivity and she sees clients and aspartate aminotransferase reaction can form citrulline, supplementing ketone ester and include total or filling in Digestive issues such as constipation and diarrhea are common side effects in the beginning. This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions. Practical considerations in the use of stable isotope labelled compounds as tracers in clinical studies. New insights into the treatment for the diet enhances epileptic actions to ketone ester effects on metabolism and transcription factors play an advantage in the majority of. What are the symptoms of ketosis and ketoacidosis? In this case, fat and carb but it could be from very poor quality of food. And it has to get burned, what are we looking at right here? Carbohydrates for training and competition. One that is vulnerable is cysteine. Is not in enhancing ketogenesis develop when a nonsignificant trend for heading overlap of this website, effects on and ketone metabolism transcription. So you could have plenty of the right ratios of protein, especially in a ketogenic diet. Ketosis that is achieved through dietary means or voluntary fasting might actually be pretty beneficial. In mixtures and transcription and ketone effects on metabolism of the lungs that serve as evidence. -

University of Warwick Institutional Repository
CORE Metadata, citation and similar papers at core.ac.uk Provided by Warwick Research Archives Portal Repository University of Warwick institutional repository: http://go.warwick.ac.uk/wrap This paper is made available online in accordance with publisher policies. Please scroll down to view the document itself. Please refer to the repository record for this item and our policy information available from the repository home page for further information. To see the final version of this paper please visit the publisher’s website. Access to the published version may require a subscription. Author(s): Hendrik Schäfer, Natalia Myronova and Rich Boden Article Title: Microbial degradation of dimethylsulphide and related C1- sulphur compounds: organisms and pathways controlling fluxes of sulphur in the biosphere Year of publication: 2010 Link to published version: http://dx.doi.org/ 10.1093/jxb/erp355 Publisher statement: This is a pre-copy-editing, author-produced PDF of an article accepted for publication in Journal of Experimental Botany following peer review. The definitive publisher-authenticated version Schäfer, H. et al. (2010). Microbial degradation of dimethylsulphide and related C1-sulphur compounds: organisms and pathways controlling fluxes of sulphur in the biosphere. Journal of Experimental Botany, Vol. 61(2), pp. 315-334 is available online at: http://jxb.oxfordjournals.org/cgi/content/full/61/2/315 Microbial degradation of dimethylsulfide and related C1-sulfur compounds: organisms and pathways controlling fluxes of sulfur in the biosphere Hendrik Schäfer*1, Natalia Myronova1, Rich Boden2 1 Warwick HRI, University of Warwick, Wellesbourne, CV35 9EF, UK 2 Biological Sciences, University of Warwick, Coventry, CV4 7AL, UK * corresponding author Warwick HRI University of Warwick Wellesbourne CV35 9EF Tel: +44 2476 575052 [email protected] For submission to: Journal of Experimental Botany 1 Abstract 2 Dimethylsulfide (DMS) plays a major role in the global sulfur cycle. -

Hydroxylamine-O -Sulfonic Acid — a Versatile Synthetic Reagent
Hydroxylamine-O -sulfonic acid — a versatile synthetic reagent Raymond G. Wallacef School of Chemistry Brunei University Uxbridge, Middlesex UBS 3PH Great Britain imidazoli nones and related derivatives are time to these various modes of reaction. discussed in the review. Many of these The uses of HOSA as a reagent are organiz preparations can be carried out in high ed below according to the different syn yield, thetic transformations that it can bring about. Hydroxylamine-Osulfonic acid, NHj-OSOjH (abbreviated to HOSA in Probably by far the most well known this article) has become in recent years and explored reactions of HOSA are commercially available. Although much animation reactions, illustrating elec fruitful chemistry has been carried out us trophilic attack by HOSA, with amination ing HOSA, to this author's knowledge, on nitrogen being the most important, there has been no systematic review in although a significant number of English* of its use as a synthetic reagent. It animations on both carbon and sulfur have is a chemically interesting compound been reported, Amination on phosphorus because of the ability of the nitrogen center also occurs. to act in the role of both nucleophile and AMINATION electrophile, dependent on circumstances, Synopsis (a) At a nitrogen atom and thus it has proved to be a reagent of Hydroxylamine-0-sulfonic acid (0 Preparation of mono- and di- great synthetic versatility. (HOSA) has only recently become widely substituted hydrazines and trisubstituied commercially available despite the fact that H,N-Nu hydrazinium salts it has proved to be a valuable synthetic reagent in preparative organic chemistry. -

Of Operation and Control Ion-Exchange Processes For
TECHNICAL REPORTS SERIES No. 78 Operation and Control Of Ion-Exchange Processes for Treatment of Radioactive Wastes INTERNATIONAL ATOMIC ENERGY AGENCY,VIENNA, 1967 OPERATION AND CONTROL OF ION-EXCHANGE PROCESSES FOR TREATMENT OF RADIOACTIVE WASTES The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GERMANY, FEDERAL NIGERIA ALBANIA REPUBLIC OF NORWAY ALGERIA GHANA PAKISTAN ARGENTINA GREECE PANAMA AUSTRALIA GUATEMALA PARAGUAY AUSTRIA HAITI PERU BELGIUM HOLY SEE PHILIPPINES BOLIVIA HUNGARY POLAND BRAZIL ICELAND PORTUGAL BULGARIA INDIA ROMANIA BURMA INDONESIA SAUDI ARABIA BYELORUSSIAN SOVIET IRAN SENEGAL SOCIALIST REPUBLIC IRAQ SIERRA LEONE CAMBODIA ISRAEL SINGAPORE CAMEROON ITALY SOUTH AFRICA CANADA IVORY COAST SPAIN CEYLON JAMAICA SUDAN CHILE JAPAN SWEDEN CHINA JORDAN SWITZERLAND COLOMBIA KENYA SYRIAN ARAB REPUBLIC CONGO, DEMOCRATIC KOREA, REPUBLIC OF THAILAND REPUBLIC OF KUWAIT TUNISIA COSTA RICA LEBANON TURKEY CUBA LIBERIA UKRAINIAN SOVIET SOCIALIST CYPRUS LIBYA REPUBLIC CZECHOSLOVAK SOCIALIST LUXEMBOURG UNION OF SOVIET SOCIALIST REPUBLIC MADAGASCAR REPUBLICS DENMARK MALI UNITED ARAB REPUBLIC DOMINICAN REPUBLIC MEXICO UNITED KINGDOM OF GREAT ECUADOR MONACO BRITAIN AND NORTHERN IRELAND EL SALVADOR MOROCCO UNITED STATES OF AMERICA ETHIOPIA NETHERLANDS URUGUAY FINLAND NEW ZEALAND VENEZUELA FRANCE NICARAGUA VIET-NAM GABON YUGOSLAVIA The Agency's Statute was approved on 26 October 1956 by the Conference on the Statute of the IAEA held at United Nations Headquarters, New York; it entered into force on 29 July 1957, The Headquarters of the Agency are situated in Vienna. Its principal objective is "to accelerate and enlarge the contribution of atomic energy to peace, health and prosperity throughout the world". © IAEA, 1967 Permission to reproduce or translate the information contained in this publication may be obtained by writing to the International Atomic Energy Agency, Kamtner Ring 11, A-1010 Vienna I, Austria. -

Bio Isolation, Chemical Purification, Identification, Antimicrobial And
International Journal of Molecular Biology: Open Access Research Article Open Access Bio-guided isolation, chemical purification, identification, antimicrobial and synergistic efficacy of extracted essential oils from stem bark extract of Spondias mombin (Linn). Abstract Volume 4 Issue 4 - 2019 The purpose of this research work is to bio-guided isolate, purify, chemical identification, antimicrobial and Synergistic efficacy of extracted essential oils from ethyl acetate extract Oludare Temitope Osuntokun,1 Gamberini of Spondias mombin. The ethyl acetate stem bark extract of Spondias mombin were air- M Cristina2 dried, chopped into smaller pieces and cold extracted exhaustively with ethylacetate. The 1Department of Microbiology, Faculty of Science, Adekunle crude extract was partitioned using various solvents and the dichloromethane fraction was Ajasin University, Nigeria concentrated and fractionated using column chromatography packed with Silica gel and 2Department of Life Sciences, University of Modena and Reggio Sephadex-LH and eluted with appropriate solvent systems accordingly. In order to obtain Emilia, Italy pure extracts, partially purified fractions were further purified. The structures of the isolated compounds were determined by using data obtained from GC-MS spectrum. The compound Correspondence: Oludare Temitope Osuntokun, Department isolated includeAspidofractinine-3-methanol, Phthalic acid, 2-ethylhexyl tetradecyl of Microbiology, Faculty of Science, Adekunle Ajasin University, ester, Phthalic acid, di (2-propylpentyl) ester), (9-(2’, 2’-Dimethylpropanoilhydrazono)- Akungba-Akoko, Ondo state, Nigeria, 3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy]fluorine) and Tere phthalic acid, dodecyl Email 2-ethylhexyl ester.These compounds have individual and synergistic activity against Gram Received: August 08, 2019 | Published: August 26, 2019 negative (E.coli), Gram-positive (Bacillus subtilis) and (Aspergillusflavus) at 10, 5, 2.5 and 1.25 g/mL.