Genetic Differentiation and Species Status of the Large-Bodied Leaf-Tailed Geckos Uroplatus Fimbriatus and U
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blumgart Et Al 2017- Herpetological Survey Nosy Komba
Journal of Natural History ISSN: 0022-2933 (Print) 1464-5262 (Online) Journal homepage: http://www.tandfonline.com/loi/tnah20 Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar Dan Blumgart, Julia Dolhem & Christopher J. Raxworthy To cite this article: Dan Blumgart, Julia Dolhem & Christopher J. Raxworthy (2017): Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar, Journal of Natural History, DOI: 10.1080/00222933.2017.1287312 To link to this article: http://dx.doi.org/10.1080/00222933.2017.1287312 Published online: 28 Feb 2017. Submit your article to this journal Article views: 23 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tnah20 Download by: [BBSRC] Date: 21 March 2017, At: 02:56 JOURNAL OF NATURAL HISTORY, 2017 http://dx.doi.org/10.1080/00222933.2017.1287312 Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar Dan Blumgart a, Julia Dolhema and Christopher J. Raxworthyb aMadagascar Research and Conservation Institute, BP 270, Hellville, Nosy Be, Madagascar; bDivision of Vertebrate Zoology, American, Museum of Natural History, New York, NY, USA ABSTRACT ARTICLE HISTORY A six month herpetological survey was undertaken between March Received 16 August 2016 and September 2015 on Nosy Komba, an island off of the north- Accepted 17 January 2017 west coast of mainland Madagascar which has undergone con- KEYWORDS fi siderable anthropogenic modi cation. A total of 14 species were Herpetofauna; conservation; found that have not been previously recorded on Nosy Komba, Madagascar; Nosy Komba; bringing the total island diversity to 52 (41 reptiles and 11 frogs). -

Reflectance of Sexually Dichromatic Uvblue Patches Varies During The
bs_bs_banner Biological Journal of the Linnean Society, 2014, 113, 556–569. With 3 figures Reflectance of sexually dichromatic UV-blue patches varies during the breeding season and between two subspecies of Gallotia galloti (Squamata: Lacertidae) MARTHA L. BOHÓRQUEZ-ALONSO and MIGUEL MOLINA-BORJA* Grupo de investigación ‘Etología y Ecología del Comportamiento’, Departamento de Biología Animal, Facultad de Biología, Universidad de La Laguna, Tenerife, Canary Islands, Spain Received 17 February 2014; revised 28 April 2014; accepted for publication 29 April 2014 Body coloration is sexually dimorphic in many vertebrate species, including lizards, in which males are often more conspicuous than females. A detailed analysis of the relative size of coloured patches and their reflectance, including the ultraviolet (UV) range, has rarely been performed. In the present work we quantified sexual dimorphism in body traits and surface area of all lateral patches from adult females and males of two subspecies of Gallotia galloti (G. g. galloti and G. g. eisentrauti). We also analysed the magnitude of sexual dichromatism in the UV-visible reflectance of such patches and the changes in patch size and brightness during the reproductive season (April–July). Males had significantly larger patch areas (relative to their snout-vent length) and higher brightness (mainly in the UV-blue range) than did females in both subspecies. The comparison of relative patch areas among months did not reach statistical significance. However, patch brightness significantly changed during the breeding season: that of the UV-blue (300–495 nm) range from lizards of the two subspecies was significantly larger in June than in April, while brightness in the 495–700 nm range in G. -

No 811/2008 of 13 August 2008 Suspending the Introduction Into the Community of Specimens of Certain Species of Wild Fauna and Flora
14.8.2008EN Official Journal of the European Union L 219/17 COMMISSION REGULATION (EC) No 811/2008 of 13 August 2008 suspending the introduction into the Community of specimens of certain species of wild fauna and flora THE COMMISSION OF THE EUROPEAN COMMUNITIES, — Accipiter erythropus, Aquila rapax, Gyps africanus, Lophaetus occipitalis and Poicephalus gulielmi from Guinea, Having regard to the Treaty establishing the European Community, — Hieraaetus ayresii, Hieraaetus spilogaster, Polemaetus bellicosus, Falco chicquera, Varanus ornatus (wild and Having regard to Council Regulation (EC) No 338/97 of ranched specimens) and Calabaria reinhardtii (wild 9 December 1996 on the protection of species of wild fauna specimens) from Togo, and flora by regulating trade therein (1), and in particular Article 19(2) thereof, — Agapornis pullarius and Poicephalus robustus from Côte d’Ivoire, After consulting the Scientific Review Group, — Stephanoaetus coronatus from Côte d’Ivoire and Togo, Whereas: — Pyrrhura caeruleiceps from Colombia; Pyrrhura pfrimeri (1) Article 4(6) of Regulation (EC) No 338/97 provides that from Brazil, the Commission may establish restrictions to the intro duction of certain species into the Community in accordance with the conditions laid down in points (a) — Brookesia decaryi, Uroplatus ebenaui, Uroplatus fimbriatus, to (d) thereof. Furthermore, implementing measures for Uroplatus guentheri, Uroplatus henkeli, Uroplatus lineatus, such restrictions have been laid down in Commission Uroplatus malama, Uroplatus phantasticus, Uroplatus Regulation (EC) No 865/2006 of 4 May 2006 laying pietschmanni, Uroplatus sikorae, Euphorbia ankarensis, down detailed rules concerning the implementation of Euphorbia berorohae, Euphorbia bongolavensis, Euphorbia Council Regulation (EC) No 338/97 of the protection duranii, Euphorbia fiananantsoae, Euphorbia iharanae, of species of wild fauna and flora by regulating trade Euphorbia labatii, Euphorbia lophogona, Euphorbia 2 therein ( ). -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -
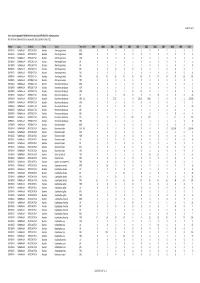
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

A Supplemental Bibliography of Herpetology in New Mexico
A Supplemental Bibliography of Herpetology in New Mexico --- Revised: 1 September 2005 --- Compiled by: James N. Stuart New Mexico Department of Game & Fish Conservation Services Division P.O. Box 25112 , Santa Fe, NM 87504-5112 and Curatorial Associate (Amphibians & Reptiles) Museum of Southwestern Biology University of New Mexico E-mail: [email protected] This document may be cited as: Stuart, J.N. 2005. A Supplemental Bibliography of Herpetology in New Mexico. Web publication (Revised: 1 September 2005): http://www.msb.unm.edu/herpetology/publications/stuart_supl_biblio.pdf Contents Section 1: Introduction and Acknowledgments Section 2: Alphabetical List of References Section 3: Index of References by Taxon or General Topic Appendix A: List of Standard English and Current Scientific Names for Amphibians and Reptiles of New Mexico Appendix B: List of State and Federally Protected Herpetofauna in New Mexico Section 1: Introduction and Acknowledgments The publication of Amphibians and Reptiles of New Mexico by W.G. Degenhardt, C.W. Painter, and A.H. Price in 1996 provided the first comprehensive review of the herpetofauna in New Mexico. Approximately 1,600 references were cited in the book and yet, as is the nature of scientific research, additional information continues to be published on the amphibian and reptile populations of this state. This supplemental bibliography was created to build on the information in Degenhardt et al. by compiling all pertinent references not included in their 1996 book or in their corrigenda to the book (Price et al. 1996). References include both peer-reviewed and non-reviewed (e.g., “gray literature”) sources such as journal and magazine articles, books, book chapters, symposium proceedings, doctoral dissertations, master’s theses, unpublished agency and contract reports, and on-line Web publications. -

COMMISSION IMPLEMENTING REGULATION (EU) 2017/1915 Of
20.10.2017 EN Official Journal of the European Union L 271/7 COMMISSION IMPLEMENTING REGULATION (EU) 2017/1915 of 19 October 2017 prohibiting the introduction into the Union of specimens of certain species of wild fauna and flora THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Regulation (EC) No 338/97 of 9 December 1996 on the protection of species of wild fauna and flora by regulating trade therein (1), and in particular Article 4(6) thereof, Whereas: (1) The purpose of Regulation (EC) No 338/97 is to protect species of wild fauna and flora and to guarantee their conservation by regulating trade in animal and plant species listed in its Annexes. The species listed in the Annexes include the species set out in the Appendices to the Convention on International Trade in Endangered Species of Wild Fauna and Flora signed in 1973 (2) (the Convention) as well as species whose conservation status requires that trade from, into and within the Union be regulated or monitored. (2) Article 4(6) of Regulation (EC) No 338/97 provides that the Commission may establish restrictions to the introduction of specimens of certain species into the Union in accordance with the conditions laid down in points (a) to (d) thereof. (3) On the basis of recent information, the Scientific Review Group established pursuant to Article 17 of Regulation (EC) No 338/97 has concluded that the conservation status of certain species listed in Annex B to Regulation (EC) No 338/97 would be seriously jeopardised if their introduction into the Union from certain countries of origin is not prohibited. -

3.4 Biological Resources
ELDORADO–IVANPAH TRANSMISSION PROJECT 3.4 BIOLOGICAL RESOURCES 1 3.4 Biological Resources 2 3 This section describes the environmental setting, regulatory setting, and potential impacts of the construction and 4 operation of the proposed project and alternatives with respect to biological resources. Information in this section is 5 largely based on the Eldorado–Ivanpah Transmission Project Biological Technical Report (EPG 2009) and the 6 Proponent’s Environmental Assessment (PEA) dated May 28, 2009, as prepared by Southern California Edison 7 (SCE, hereafter referred to as the applicant). Details on locations of the EITP facilities, rights-of-way (ROWs), extra 8 workspaces, and staging areas can be found in Chapter 2. Chapter 2 also provides a detailed description of 9 construction, operation, and maintenance techniques used for the proposed project and alternatives to the proposed 10 project. Comments received from the general public and resource agencies during the scoping process are evaluated 11 and addressed as well in Section 3.4.3, ―Impact Analysis.‖ 12 13 3.4.1 Environmental Setting 14 15 The EITP is located within the Eldorado and Ivanpah valleys in southern Clark County, Nevada, and in southeastern 16 California. The project would cross public and privately owned lands (see Section 3.9, ―Land Use, Agricultural 17 Resources, and Special Management Areas‖). Most of the lands that would be crossed by the transmission line in 18 California are administered by the BLM. Small segments would cross private parcels at Nipton, California, and in the 19 vicinity of the Mountain Pass Substation. Similarly, the EITP in Nevada is predominantly situated on BLM lands, but 20 private lands would be crossed near the Eldorado Substation and possibly at Primm, Nevada. -

Lizards & Snakes: Alive!
LIZARDSLIZARDS && SNAKES:SNAKES: ALIVE!ALIVE! EDUCATOR’SEDUCATOR’S GUIDEGUIDE www.sdnhm.org/exhibits/lizardsandsnakeswww.sdnhm.org/exhibits/lizardsandsnakes Inside: • Suggestions to Help You Come Prepared • Must-Read Key Concepts and Background Information • Strategies for Teaching in the Exhibition • Activities to Extend Learning Back in the Classroom • Map of the Exhibition to Guide Your Visit • Correlations to California State Standards Special thanks to the Ellen Browning Scripps Foundation and the Nordson Corporation Foundation for providing underwriting support of the Teacher’s Guide KEYKEY CONCEPTSCONCEPTS Squamates—legged and legless lizards, including snakes—are among the most successful vertebrates on Earth. Found everywhere but the coldest and highest places on the planet, 8,000 species make squamates more diverse than mammals. Remarkable adaptations in behavior, shape, movement, and feeding contribute to the success of this huge and ancient group. BEHAVIOR Over 45O species of snakes (yet only two species of lizards) An animal’s ability to sense and respond to its environment is are considered to be dangerously venomous. Snake venom is a crucial for survival. Some squamates, like iguanas, rely heavily poisonous “soup” of enzymes with harmful effects—including on vision to locate food, and use their pliable tongues to grab nervous system failure and tissue damage—that subdue prey. it. Other squamates, like snakes, evolved effective chemore- The venom also begins to break down the prey from the inside ception and use their smooth hard tongues to transfer before the snake starts to eat it. Venom is delivered through a molecular clues from the environment to sensory organs in wide array of teeth. -

The Biogeography of Mitochondrial and Nuclear Discordance in Animals
Molecular Ecology (2012) doi: 10.1111/j.1365-294X.2012.05664.x INVITED REVIEWS AND META-ANALYSES The biogeography of mitochondrial and nuclear discordance in animals DAVID P. L. TOEWS* and ALAN BRELSFORD† *Department of Zoology and Biodiversity Research Centre, University of British Columbia, 6270 University Blvd., Vancouver, BC V6T 1Z4, Canada, †Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland Abstract Combining nuclear (nuDNA) and mitochondrial DNA (mtDNA) markers has improved the power of molecular data to test phylogenetic and phylogeographic hypotheses and has highlighted the limitations of studies using only mtDNA markers. In fact, in the past decade, many conflicting geographic patterns between mitochondrial and nuclear genetic markers have been identified (i.e. mito-nuclear discordance). Our goals in this synthesis are to: (i) review known cases of mito-nuclear discordance in animal systems, (ii) to summarize the biogeographic patterns in each instance and (iii) to identify common drivers of discordance in various groups. In total, we identified 126 cases in animal systems with strong evidence of discordance between the biogeographic patterns obtained from mitochondrial DNA and those observed in the nuclear genome. In most cases, these patterns are attributed to adaptive introgression of mtDNA, demographic disparities and sex-biased asymmetries, with some studies also implicating hybrid zone movement, human introductions and Wolbachia infection in insects. We also discuss situations where divergent mtDNA clades seem to have arisen in the absence of geographic isolation. For those cases where foreign mtDNA haplotypes are found deep within the range of a second taxon, data suggest that those mtDNA haplotypes are more likely to be at a high frequency and are commonly driven by sex-biased asymmetries and ⁄ or adaptive introgression. -

Characterization of Five Complete Cyrtodactylus Mitogenome Structures Reveals Low Structural Diversity and Conservation of Repeated Sequences in the Lineage
Characterization of five complete Cyrtodactylus mitogenome structures reveals low structural diversity and conservation of repeated sequences in the lineage Prapatsorn Areesirisuk1,2,3, Narongrit Muangmai3,4, Kirati Kunya5, Worapong Singchat1,3, Siwapech Sillapaprayoon1,3, Sorravis Lapbenjakul1,3, Watcharaporn Thapana1,3,6, Attachai Kantachumpoo1,3,6, Sudarath Baicharoen7, Budsaba Rerkamnuaychoke2, Surin Peyachoknagul1,8, Kyudong Han9 and Kornsorn Srikulnath1,3,6,10 1 Laboratory of Animal Cytogenetics and Comparative Genomics (ACCG), Department of Genetics, Faculty of Science, Kasetsart University, Bangkok, Thailand 2 Human Genetic Laboratory, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand 3 Animal Breeding and Genetics Consortium of Kasetsart University (ABG-KU), Kasetsart University, Bangkok, Thailand 4 Department of Fishery Biology, Faculty of Fisheries, Kasetsart University, Bangkok, Thailand 5 Nakhon Ratchasima Zoo, Nakhon Ratchasima, Thailand 6 Center for Advanced Studies in Tropical Natural Resources, National Research University-Kasetsart University (CASTNAR, NRU-KU, Thailand), Kasetsart University, Bangkok, Thailand 7 Bureau of Conservation and Research, Zoological Park Organization under the Royal Patronage of His Majesty the King, Bangkok, Thailand 8 Department of Biology, Faculty of Science, Naresuan University, Phitsanulok, Thailand 9 Department of Nanobiomedical Science & BK21 PLUS NBM Global Research Center for Regenerative Medicine, Dankook University, Cheonan, Republic of Korea 10 Center of Excellence on Agricultural Biotechnology: (AG-BIO/PERDO-CHE), Bangkok, Thailand ABSTRACT Submitted 30 July 2018 Accepted 15 November 2018 Mitochondrial genomes (mitogenomes) of five Cyrtodactylus were determined. Their Published 13 December 2018 compositions and structures were similar to most of the available gecko lizard Corresponding author mitogenomes as 13 protein-coding, two rRNA and 22 tRNA genes.