Journal of Environmental Analysis and Progress V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
1 B 2 C 3 B 4 D 5 D
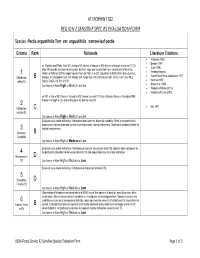
ATTACHMENT SS2 REGION 2 SENSITIVE SPECIES EVALUATION FORM Species: Pectis angustifolia Torr. var. angustifolia narrow-leaf pectis Criteria Rank Rationale Literature Citations • Anderson 1950 • Barkley 1991 se (Goshen and Platte Cos) WY, e into w NE; thence s through w KS; thence w through e and sw (?) CO. • Clark 1996 Most NE records are from the sw corner, but Keil maps one record from the n-central part of the state. 1 Weber & Wittman (2001b) report species from La Plata in w CO. [Vouchers at KANU from Baca, Lincoln, • Freeman in prep. • Great Plains Flora Association 1977 Distribution B Morgan, and Sedgwick Cos, CO; Meade and Trego Cos, KS; and Deuel, Keith, and Lincoln Cos, NE.] within R2 Status: G4G5; KS SH; WY S1 • Hartman 1997 • Shaw et al. 1989 Confidence in Rank High or Medium or Low • Weber & Wittman 2001a • Weber & Witman 2001b se WY, e into w NE; thence s through w KS; thence s w-most TX into n Mexico; thence n throughout NM; thence n through e CO, and w through n AZ and se-most UT. 2 • Keil 1977 Distribution C outside R2 Confidence in Rank High or Medium or Low Evaluator was unable to find any information about species’ dispersal capability. Seed is equipped with a pappus and may be dispersed by wind or perhaps water, during heavy rains. Dispersal is probably limited by 3 habitat requirements. Dispersal B Capability Confidence in Rank High or Medium or Low Evaluator was unable to find any information on species’ abundance within R2. Species does not appear to 4 be particularly abundant where encountered in CO and populations may face local extinction. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Lectotypification of Pectis L. (Asteraceae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@CalPoly LECTOTYPIFICATION OF PECTIS L. (ASTERACEAE) David J. Keil'Kei/ t Linnaeus (I(1759a)759a) described Pectis and included within it two species, P. ciliaris and P. linifolia. In 1913 Britton and Brown designated P. ciliaris as the lectotype for Pectis. This lectotypification was accepted by the editors of Index Nominum Genericorum (Plantarum) (FaIT(Farr et aI.,al., 1979). I will present evidence below that the lectotypification of Pectis by Britton and Brown was superfluous and that Pectis was actually lectotypified over 90 years earlier by Cassini. Britton and Brown as adherents of the American Code of Botanical Nomenclature (Anonymous, 1907) followed the mechanical practice of designating "the first binomial species in order" as the lectotype of a genus. This appears to have been the method of lectotypification used for Pectis. This practice is in conflict with the provisions of Article 8 of the International Code of Botanical NomenNomen- clature (Voss et aI.,al., 1983). The choice of Pectis ciliaris as lectotype is also in conflict with the Linnaean protologue of Pectis. Linnaeus (I(1759a)759a) included both a diagnosis (p. 1221) and a description (p. 1376) for Pectis. The diagnosis is very brief: PECTIS. Recept. nudum. Pappus aristatus. Cal. 5-phyllus, cylindricus. Flosculi radiantes 5. Of the features described, the receptacle, the involucreinvolucre [caL][cal.] and the number of ray florets are the same for both P. ciliaris and P. linifolia.linifolia. However, only P. linifolialinifolia has an aristate pappus. -

The C4 Plant Lineages of Planet Earth
Journal of Experimental Botany, Vol. 62, No. 9, pp. 3155–3169, 2011 doi:10.1093/jxb/err048 Advance Access publication 16 March, 2011 REVIEW PAPER The C4 plant lineages of planet Earth Rowan F. Sage1,*, Pascal-Antoine Christin2 and Erika J. Edwards2 1 Department of Ecology and Evolutionary Biology, The University of Toronto, 25 Willcocks Street, Toronto, Ontario M5S3B2 Canada 2 Department of Ecology and Evolutionary Biology, Brown University, 80 Waterman St., Providence, RI 02912, USA * To whom correspondence should be addressed. E-mail: [email protected] Received 30 November 2010; Revised 1 February 2011; Accepted 2 February 2011 Abstract Using isotopic screens, phylogenetic assessments, and 45 years of physiological data, it is now possible to identify most of the evolutionary lineages expressing the C4 photosynthetic pathway. Here, 62 recognizable lineages of C4 photosynthesis are listed. Thirty-six lineages (60%) occur in the eudicots. Monocots account for 26 lineages, with a Downloaded from minimum of 18 lineages being present in the grass family and six in the sedge family. Species exhibiting the C3–C4 intermediate type of photosynthesis correspond to 21 lineages. Of these, 9 are not immediately associated with any C4 lineage, indicating that they did not share common C3–C4 ancestors with C4 species and are instead an independent line. The geographic centre of origin for 47 of the lineages could be estimated. These centres tend to jxb.oxfordjournals.org cluster in areas corresponding to what are now arid to semi-arid regions of southwestern North America, south- central South America, central Asia, northeastern and southern Africa, and inland Australia. -

Numerical Taxonomic Study of Some Tribes of Compositae (Subfamily Asteroideae) from Egypt
Pak. J. Bot., 43(1): 171-180, 2011. NUMERICAL TAXONOMIC STUDY OF SOME TRIBES OF COMPOSITAE (SUBFAMILY ASTEROIDEAE) FROM EGYPT A. K. OSMAN Department of Botany, Faculty of Science, South Valley University, Qena, Egypt. Abstract A systematic study of 25 taxa belonging to 12 genera of tribes Gnaphalieae, Helenieae, Plucheeae and Senecioneae of Compositae from Egypt was conducted by means of numerical analysis based on 19 main pollen grains characters. On the basis of UPGMA (Unpaired Group Method off Averaging) clustering and PCO (Principal Component Analysis), two main groups and five subgroups are recognized. Introduction The Compositae (Asteraceae) is the largest family of plants, comprises of 1590 genera and around 23,600 known species arranged in 3 subfamilies, Asteroideae, Cichorioideae and Barnadesioideae and 17 tribes (Bremer, 1994; Bremer & Jansen 1992). Tribe Gnaphalieae is one of the largest in the family, with more than 180 genera and 2000 species. Most Gnaphalieae are characterized by a two-layered pollen sexine with an outer baculae and an inner perforated layer. The Gnaphalieae are subdivided into five subtribes (Anderberg, 1991a). In subtribe Gnaphaliinae, the two largest genera are Helichrysum and Gnaphalium, with hundreds of species and with many closely related segregate genera. Classification problems within the tribe are dominated by the difficulties in generic delimitation of Helichrysum and Gnaphalium. The Helenieae comprise a little more than 800 species in 110 genera, are often herbs. Some species have become naturalized as weeds in most parts of the world, eg., Tagetes minuta L. (Bierner, 1989). The widespread, familiar genera of the Helenieae are Flaveria, Helenium, Pectis and Tagetes. -

(Tageteae, Asteraceae), a C4 Genus of the Neotropics
6 LUNDELLIA DECEMBER, 2016 MOLECULAR PHYLOGENY OF PECTIS (TAGETEAE,ASTERACEAE), A C4 GENUS OF THE NEOTROPICS, AND ITS SISTER GENUS POROPHYLLUM Debra R. Hansen,1 Robert K. Jansen,1 Rowan F. Sage,2 Jose´ Luis Villasen˜or,3 and Beryl B. Simpson1 1Department of Integrative Biology, The University of Texas at Austin, 205 West 24th Street, Austin, Texas 78712, USA, email: [email protected] 2Department of Ecology & Evolutionary Biology, University of Toronto, 25 Willcocks Street, Room 3055, Toronto, Ontario, Canada M5S 3B2 3Departamento de Bota´nica, Instituto de Biologı´a, Universidad Nacional Auto´noma de Me´xico, Apartado Postal 70-233, 04510 Me´xico, D.F. Mexico Abstract: Pectis is a genus of 690 xeric adapted New World species. Previous molecular phylogenetic studies showed Pectis closely related to Porophyllum, and one analysis resolved Porophyllum species nested within Pectis. Some Pectis species are known to use C4 photosynthesis. Here we investigate the phylogeny of Pectis and Porophyllum, examine the ploidy levels and geographical distribution of Pectis species in light of its phylogeny, and infer the origin and extent of C4 photosynthesis in both genera. Chloroplast and ITS data from 78 Pectis and 22 Porophyllum species were used to test the monophyly of Pectis and its previously described sections. Carbon isotope data were obtained to infer the photosynthetic pathway of 80 species, and the results mapped on the inferred phylogenies to determine the timing and pattern of evolution of the C4 pathway. The ITS dataset supports a monophyletic Pectis sister to a monophyletic Porophyllum, while the chloroplast dataset places two Porophyllum species sister to a combined Pectis+Porophyllum clade. -

Las Formaciones Vegetales De Los Hemiagriófitos Son Pocas, Dado Que
CAP 6 Especies vegetales sinántropas alóctonas de Cuba. 263 Las formaciones vegetales de los hemiagriófitos son pocas, dado que estas plantas dependen de la vegetación secundaria para su supervivencia, como es el caso del Bosque Secundario y del Matorral Secundario, así como de las Sabanas Seminaturales y de las Sabanas Antrópicas, pero están ausentes de las vegetaciones ruderal y segetal pues en los alrededores de las comunidades humanas y en los cultivos se les hace una guerra sin cuartel, de exterminio total. Sin embargo, si esta guerra no existiera (lo cual es imposible) los hemiagriófitos son totalmente capaces de invadir las áreas en que se hallan los hemiagriófitos-epecófitos y los epecófitos. Las familias están representadas, tanto en formaciones vegetales arbóreas secundarias y matorrales degradados como en herbazales y complejos de vegetación sometidos a una acción antrópica mediana a fuerte, y abarcan desde los ecosistemas litorales o sublitorales hasta altitudes superiores a los 1200 m. Los hemiagriófitos son especies invasoras de formaciones vegetales secundarias, cuyo número de individuos y poblaciones crece explosivamente ante la acción natural o antrópica, y en las cuales la intensidad luminosa juega un papel decisivo. El comportamiento generalmente agresivo de los hemiagriófitos los convierte en las invasoras más peligrosas del archipiélago cubano conjuntamente con los hemiagriófitos-epecófitos y los epecófitos. Todas las medidas que se tomen para el control y manejo de estos tres grupos o phydia serán siempre insuficientes. Afortunadamente, dado que los hemiagriófitos son especies de larga vida y madurez sexual rara vez precoz, no pueden atacar las áreas ocupadas por cultivos permanentes, ya que las labores que se llevan a cabo erradican a estas agresivas invasoras en las fases más débiles de su largo ciclo de vida: las de semilla y postura. -

New Species of Pectis (Asteraceae) from the West Indies, Mexico, and South America
NEW SPECIES OF PECTIS (ASTERACEAE) FROM THE WEST INDIES, MEXICO, AND SOUTH AMERICA DAVID J. KEIL Keil, David J. (Biological Sciences Department, California Polytechnic State University, San Luis Obispo, CA 93407). New species ofPeetis (Asteraceae) from the West Indies, Mexico, and South America. Brittonia 36: 74-80. 1984.- Four new species of Peetis are described: P. ericifolia from Barbuda, P. luckoviae from west-central Mexico, P. arida from Ecuador and Peru, and P. cajamarcana from Peru. Chromosome counts for P. erieifolza (n = 48) and P. luekoviae (n = 12) are presented. Relationships of the newly described taxa are discussed. Recent field and herbarium research on the systematics of Pectis (Asteraceae) has resulted in the discovery of four previously undescribed species. Pectis ericifolia Keil, sp. nov. A P. tenuieaule Urban et P. humifusa Sw. lamina cujusque phyllarii dorso sub apice macula ru bescente ornata, ligula cujusque radii dorso vinaceo-maculata, radiorum numero variabile, pappo reducto et chromosomatum numero octoploideo differt. Ulterius a P. humifusa minore flosculorum disci numero abstat. Non-scented fibrous-rooted annual, much-branched from base, mat-forming, seldom radicant. Stems 4-25 cm long, 0.2-1 mm diameter, dark purplish, densely leafy with internodes mostly 0.8-2.5 mm long, hirtellous in decurrent lines with erect whitish trichomes 0.1-0.2 mm long. Leaves linear oblanceolate, 8-20 mm long, 2-4 mm broad, at apex rounded and usually mucronulate, glabrous on both surfaces, minutely antrorsely scaberulous on margins toward apex with trichomes 0.1-0.2 mm long, hirtellous proximally on margins with trichomes like those on the stem, ciliate on proximal ~_1;; with 3-5 pairs ofbristles 1-2 mm long, punctate on undersurface with submarginal and scattered circular glands 0.1-0.2 mm di ameter. -

Vascular Plant Species of the Comanche National Grassland in United States Department Southeastern Colorado of Agriculture
Vascular Plant Species of the Comanche National Grassland in United States Department Southeastern Colorado of Agriculture Forest Service Donald L. Hazlett Rocky Mountain Research Station General Technical Report RMRS-GTR-130 June 2004 Hazlett, Donald L. 2004. Vascular plant species of the Comanche National Grassland in southeast- ern Colorado. Gen. Tech. Rep. RMRS-GTR-130. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 36 p. Abstract This checklist has 785 species and 801 taxa (for taxa, the varieties and subspecies are included in the count) in 90 plant families. The most common plant families are the grasses (Poaceae) and the sunflower family (Asteraceae). Of this total, 513 taxa are definitely known to occur on the Comanche National Grassland. The remaining 288 taxa occur in nearby areas of southeastern Colorado and may be discovered on the Comanche National Grassland. The Author Dr. Donald L. Hazlett has worked as an ecologist, botanist, ethnobotanist, and teacher in Latin America and in Colorado. He has specialized in the flora of the eastern plains since 1985. His many years in Latin America prompted him to include Spanish common names in this report, names that are seldom reported in floristic pub- lications. He is also compiling plant folklore stories for Great Plains plants. Since Don is a native of Otero county, this project was of special interest. All Photos by the Author Cover: Purgatoire Canyon, Comanche National Grassland You may order additional copies of this publication by sending your mailing information in label form through one of the following media. -

Species Risk Assessment
Ecological Sustainability Analysis of the Kaibab National Forest: Species Diversity Report Ver. 1.2 Prepared by: Mikele Painter and Valerie Stein Foster Kaibab National Forest For: Kaibab National Forest Plan Revision Analysis 22 December 2008 SpeciesDiversity-Report-ver-1.2.doc 22 December 2008 Table of Contents Table of Contents............................................................................................................................. i Introduction..................................................................................................................................... 1 PART I: Species Diversity.............................................................................................................. 1 Species List ................................................................................................................................. 1 Criteria .................................................................................................................................... 2 Assessment Sources................................................................................................................ 3 Screening Results.................................................................................................................... 4 Habitat Associations and Initial Species Groups........................................................................ 8 Species associated with ecosystem diversity characteristics of terrestrial vegetation or aquatic systems ...................................................................................................................... -

Synopsis of the Florida Species of Pectis (Asteraceae)
SYNOPSIS OF THE FLORIDA SPECIES OF PECTIS (ASTERACEAE) DAVID J. KEIL Biological Sciences Department California Polytechnic State University San Lllis Obispo, CA 93407, U.S.A. ABSTRACT A key, descriptions, revised nomenclature, range statements and maps ate presented for the species of Pectis known to occur in Florida. A new combination, Peetis glaucescens, is published for the plant formerly known as P. leptocephala. A naturally occurring triploid interspecific hybrid, Peetis X floridana (P. glallcescens X P. prostrata), is described and illustrated. Four species of Pertis (Asteraceae) have been reported to occur in Florida: p. humi/usa, P. leptorephala, P. lineari/olia, and P. prostrata (Fernald 1897; Rydberg 1916; Small 1933; Keil 1975; Long and Lakela 1976; Cronquist 1980; Wunderlin 1982). Systematic investigations of Pertis necessitate some taxonomic changes for the species occurring in Florida. An examina tion of type specimens deposited in the herbarium of the Museum National d'Histoire Naturelle (P) revealed that the basionym of P. leptocephala is predated by an earlier available epithet and that a nomenclatural change is required. Recent field studies have revealed the presence of a heretofore unrecognized natural interspecific hybrid that closely resembles Pertis lineari/olia. The range of several species is greater than indicated in local and regional manuals (e.g., Anderson 1984). PECnS 1., Syst. Nat. Ed. 10. 1221. 1759. TYPE: P. linifolia L. Tap-rooted or fibrous-rooted annual or perennial herbs. Stems prostrate to erect, often several arising together from the base, straw-colored to deep purplish brown, often diffusely branched, glabrous to puberulent. Leaves opposite, linear to oblanceolate, connected at the base by a narrow connate rim, proximally ciliate with slender bristles, dotted on the undersurface with pellucid glands containing scented oils or unscented compounds, glabrous or minutely puberulent on the margin and midvein. -

(Asteraceae: Tageteae) from South America
Two New Species of Pectis (Asteraceae: Tageteae) from South America David ]. J. Keil Biological Sciences Department, California Polytechnic State University, San Luis Obispo, California 93407, U.S.A. [email protected] ABSTRACT. Pectis hassleri and P. pumila are new andandmore morenumerousnumerous thanthan those thoseof of thethe abaxialabaxialsursur- species, the former from the Gran Chaco area of face,face, minutelyminutelyciliolateciliolate nearnear thethe base,base, otherwiseotherwise Paraguay and the latter from southwestern Ecuador glabrous.glabrous.CapitulaCapitula long-peduncled,long-peduncled, solitarysolitary oror inin and northwestern Peru. Pectis hassleri differs from open,open, few-headedfew-headedcymes,cymes, ca.ca. 38-38- toto 75-flowered;75-flowered; p.P. odorata by leaves that are glandular-punctate on pedunclespeduncles3-113-11 cmcm long,long,slightly slightlyclavate,clavate, bearingbearing the adaxial as well as the abaxial surfaces, by lonlon- 33 toto7 7 linear-acuminatelinear-acuminatescale-likescale-like bractletsbractlets 3-63-6 mmmm ger and wider ligules of the ray florets, and by ray longlongor or thethe proximalproximalbractbract largerlarger andand leaflike. leaflike.InIn- pappi of awns and shorter bristles. Pectis pumila volucresvolucrescampanulate;campanulate; phyllariesphyllaries 88 (to(to 10),10), oblanoblan- differs from the closely related P.R arida by its wider ceolateceolate toto obovate,obovate,6-8.5S8.5 Xx 2-3.52-3.5 mm,mm, broadlybroadly leaves and by its sessile or subsessile capitula with overlapping,overlappingesubacute,subacute, weaklyweakly convex, convex,proximally proximally campanulate involucres and obovate phyllaries. A roundedroundedandand gibbous, gibbous,indurate-keeledindurate-keeled inin thethe proxprox- 2 hexaploid chromosome count of 2n = 3636111 is newly imalimallh-1/2-2/3, /3, narrowlynarrowlyhyaline-hyaline- oror purple-margined purple-marginedinin reported for P.