Characterizing the Flow Regime in Brook Trout (Salvelinus Fontinalis) Incubation Habitats and the Implications for Management in a Hydro-Regulated River
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outlook for Break-Up of Ice on the St.Lawrence Seaway & Lake Erie Issued by the Canadian Ice Service
OUTLOOK FOR BREAK-UP OF ICE ON THE ST.LAWRENCE SEAWAY & LAKE ERIE ISSUED BY THE CANADIAN ICE SERVICE Issued by Canadian Ice Service of Environment and Climate Change Canada Prepared for The Saint-Lawrence Seaway Management Corporation 2 February 2021 CURRENT CONDITIONS Average temperatures over Lake Erie, Lake Ontario, and the Seaway have been well above normal values consistently since the start of the ice season in early November until near the end of January. At the end of January, temperatures were near to below normal. The highest temperature anomalies occurred in the second half of December and first half of January. The table below indicates the departure from normal temperatures at specific locations, on a bi- weekly basis, for the period from mid-November to the end of January: November December January 16 Nov. – 16-30 31 Jan 01-15 16-31 01-15 16-31 Montreal +2.0 ºC +4.0ºC +4.1ºC +6.8ºC +0.9ºC +3.6ºC Kingston +2.8ºC +2.3ºC +3.2ºC +5.4ºC +1.0ºC +2.9ºC Windsor +1.5ºC +1.3ºC +2.0ºC +4.3ºC +1.4ºC +2.2ºC Table 1: Departure from normal temperatures With the warm conditions experienced through November across the southern Great Lakes (Erie and Ontario), no ice formed in November. The first ice was recorded on December 16th in the Bay of Quinte and in sheltered bays in eastern Lake Ontario, which was a week earlier than normal. In Lake Erie, the first ice was seen a week and a half later, on December 26th, in line with climatology. -

NIAGARA RIVER WATERSHED MANAGEMENT PLAN (Phase 1)
ATLAS NIAGARA RIVER WATERSHED MANAGEMENT PLAN (Phase 1) September 2013 During the development of the Niagara River Watershed Management Plan (Phase 1), an impressive collection of existing plans, studies, reports, data, information and maps were gathered and reviewed to help establish the overall physical, biological and ecological conditions of the Niagara River Watershed. This Atlas is a full assembly of these resources, providing a comprehensive record of previous watershed efforts utilized in the watershed management planning process. NIAGARA RIVER WATERSHED MANAGEMENT PLAN Developed By: 1250 Niagara Street Buffalo, NY 14213 Buffalo Niagara RIVERKEEPER® is a community‐based organization dedicated to protecting the quality and quantity of water, while connecting people to water. We do this by cleaning up pollution from our waterways, restoring fish and wildlife habitat, and enhancing public access through greenways that expand parks and open space. In Conjunction with: 2919 Delaware Ave. 478 Main Street Kenmore, NY 14217 Buffalo, NY 14202 Financial support for the development of this Atlas and the Niagara River Watershed Management Plan (Phase 1) is from the New York State Department of State with funds provided under Title 11 of the Environmental Protection Fund. For more information on the Niagara River Watershed Management Plan (Phase 1), or to become involved in our regional watershed’s protection and restoration, visit Buffalo Niagara RIVERKEEPER® online at www.bnriverkeeper.org. For more information regarding watershed planning in New York State, visit the NYS Department of State’s website at www.nyswaterfronts.com/watershed_home.asp. 1 NIAGARA RIVER WATERSHED MANAGEMENT PLAN 2 NIAGARA RIVER WATERSHED MANAGEMENT PLAN Atlas Layout The Atlas has been divided into the following sections that correspond with the watershed as a whole and the 11 sub-watersheds that make up the Niagara River watershed. -

French River Provincial Park Management Plan
French River Provincial Park Management Plan November 1993 C 1993 Queen’s Printer for Ontario Printed in Ontario, Canada For more information or additional copies of this publication contact: Ontario Ministry of Natural Resources, 199 Larch St., Sudbury, Ontario, P3E 5P9. Telephone (705) 675-4120 Approval Statement We are pleased to approve this revised Management Plan as official policy for the French River Provincial Park. The plan reflects the Ministry of Natural Resources’ intent to protect the natural and cultural features of French River Provincial Park while maintaining high quality opportunities for outdoor recreation and heritage appreciation for Ontario’s residents and visitors. The Park’s original management plan was written in 1986 when the French River became the first Canadian Heritage River in Canada. French River Provincial Park was established in 1989. The River is unique within Ontario’s Provincial Park system as the largest waterway park draining into the Great Lakes. A drainageway in this part of the Canadian Shield proceeded the last glacial period, which began 45, 000 years ago. The Park contains an extensive bedrock delta and a fault controlled main river channel. Shoreline habitats from Georgian Bay to Lake Nipissing including wetlands and upland forests. The waterway is an ancient travel route, which has been used since cultures inhabited this part of Ontario 6,000 years ago. It has been an important recreation and tourism area for 100 years. Today the scenic quality of the River continues to be an outstanding attraction for visitors. The policies in this Plan are consistent with new implementation details on province wide policies for park planning and management. -

Checklist of Fishes of Thunder Bay District, Ontario
Thunder Bay Field Naturalists Checklist of Fish es of Thunder Bay District , Ontario 31 December 2019 Introduction This first edition of Checklist of Fishes of Thunder Bay District adds to existing checklists prepared by members of the Thunder Bay Field Naturalists (TBFN) covering other vertebrate taxa (mammals, birds, reptiles & amphibians), as well vascular plants, butterflies, and odonates. As with these other checklists, it covers the official judicial District of Thunder Bay (Figure 1). The District extends from the eastern border of Quetico Provincial Park east to White River, and from the international border north to Lake St. Joseph and the Albany River. Much of the District (60%) is within the Great Lakes watershed, with the remaining draining into the Arctic Ocean either north via the Hudson Bay Lowlands, or west via Rainy Lake/Lake of the Woods and the Nelson River watershed. Figure 1. Judicial District of Thunder Bay with primary watersheds and protected areas. 2 The fish species of the Thunder Bay District mostly reflect post-glacial colonization, modified by more recent ecological and anthropogenic influences. The Wisconsinan ice mass began to retreat north of Lake Superior circa 10,700 BP (Farrand and Drexler 1985), allowing fish to initially colonize the Thunder Bay area (Momot and Stephenson 1996). The Marquette advance circa 9900 BP likely wiped out these early colonizers, but its retreat around 9700 BP allowed many species access from glacial refugia in the Mississippi River basin to the south (Mandrak and Crossman 1992b; Stephenson and Momot 1994). Some species invaded from the east via the outlet of Lake Minong and Lake Superiors’ other post-glacial predecessors. -

Controlled Flow, Temperature and Brook Trout (Salvelinus Fontinalis Mitchell) Fry Emergence on the Aguasabon River, Terrace Bay, Ontario, 2011-2016
Lakehead University Knowledge Commons,http://knowledgecommons.lakeheadu.ca Electronic Theses and Dissertations Undergraduate theses 2017 Controlled flow, temperature and brook trout (Salvelinus fontinalis Mitchell) fry emergence on the Aguasabon River, Terrace Bay, Ontario, 2011-2016 Hauser, Allison Hanna http://knowledgecommons.lakeheadu.ca/handle/2453/4367 Downloaded from Lakehead University, KnowledgeCommons i CONTROLLED FLOW, TEMPERATURE AND BROOK TROUT (Salvelinus fontinalis Mitchell) FRY EMERGENCE ON THE AGUASABON RIVER, TERRACE BAY, ONTARIO, 2011 -2016 By Allison Hanna Hauser Source: Ontario Power Generation 2016 Source: Parks Canada 2015 Faculty of Natural Resources Management Lakehead University Thunder Bay Ontario April 2017 i CONTROLLED FLOW, TEMPERATURE AND BROOK TROUT ( Salvelinus fontinalis Mitchell) FRY EMERGENCE ON THE AGUASABON RIVER, TERRACE BAY, ONTARIO, 2011-2016 By Allison Hanna Hauser An undergraduate thesis submitted in partial fulfillment of the requirements for the degree of Honours Bachelor of Environmental Management Faculty of Natural Resources Management Lakehead University April 2017 _____________________________ ___________________________ Major Advisor - Dr. Brian McLaren Second Reader – Ray Tyhuis iii A CAUTION TO THE READER This HBEM thesis has been through a semi -formal process of review and comment by one faculty member. It has also been through the same process with a biologist of the Ministry of Natural Resourc es and Forestry (MNRF). It is made available for loan by the Faculty of Natural Resources Management for the purpose of advancing the practice of professional and scientific natural resources manag ement. The reader should be aware that opinions and conclusions expressed in this document are those of the student and do not necessarily reflect the opinions of the thesis supervisor, the faculty, Lakehead University, or the MNRF. -
M I C H I G a N O N T a R
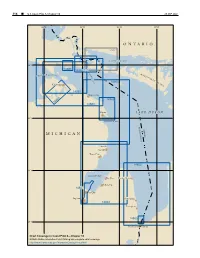
314 ¢ U.S. Coast Pilot 6, Chapter 10 26 SEP 2021 85°W 84°W 83°W 82°W ONTARIO 2251 NORTH CHANNEL 46°N D E 14885 T 14882 O U R M S O F M A C K I N A A I T A C P S T R N I A T O S U S L A I N G I S E 14864 L A N Cheboygan D 14881 Rogers City 14869 14865 14880 Alpena L AKE HURON 45°N THUNDER BAY UNITED ST CANADA MICHIGAN A TES Oscoda Au Sable Tawas City 14862 44°N SAGINAW BAY Bay Port Harbor Beach Sebewaing 14867 Bay City Saginaw Port Sanilac 14863 Lexington 14865 43°N Port Huron Sarnia Chart Coverage in Coast Pilot 6—Chapter 10 NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 26 SEP 2021 U.S. Coast Pilot 6, Chapter 10 ¢ 315 Lake Huron (1) Lawrence, Great Lakes, Lake Winnipeg and Eastern Chart Datum, Lake Huron Arctic for complete information.) (2) Depths and vertical clearances under overhead (12) cables and bridges given in this chapter are referred to Fluctuations of water level Low Water Datum, which for Lake Huron is on elevation (13) The normal elevation of the lake surface varies 577.5 feet (176.0 meters) above mean water level at irregularly from year to year. During the course of each Rimouski, QC, on International Great Lakes Datum 1985 year, the surface is subject to a consistent seasonal rise (IGLD 1985). -

Task 3 Neil Fulton
REPORT ON POTENTIAL MEASURES TO ALLEVIATE PROBLEMS CREATED BY CtIFWNT HIGH LAKE LEVELS TASK 3 DIVERSIONS MANAGEMENT ,.l -. TO THE INTERNATIONAL JOINT CCXMISSICPJ FROM TASK GROUP MEMBERS GERALD MACMILLXY DON LEONARD RM; GOLDING BILL DALEY MAC ODELL BILL ERDLE PETER YEE . NEIL FULTON SEPTEMBER 1987 EXExmTIVE SUMMARY The Governments of Canada and the United States requested, in August 1986, that the International Joint 'Commission study methods of alleviating the adverse consequences of fluctuating Great Lakes water levels. Part of this study entailed an immediate short-term investigation of methods of alleviating the present high water level crisis. One of the elements of the short-term investigation was a study of diversions management and the results are contained herein. The theoretical maximum flow through the Welland Canal in an extreme crisis situation was estimated to be 12,000 cubic feet per second (cfs). The most significant physical impacts of this maximum flow would be the complete disruption of navigation between Lake Ontario and the upper lakes and the possibility of major damage to the canal itself. A reduction in Lake Erie levels of 1.3 inches would be achieved. The Ogoki and Long Lac Diversions can be closed completely but, because of significant social impacts associated with the closure of the Long Lac Diversion, a.scenario involving a combined annual diversion flow of 800 cfs was investigated to determine the benefits foregone by maintaining the log-driving operation from Long Lake. The most significant physical impacts were the direct loss of 2,500 jobs in the Terrace Bay - Longlac area resulting from the closure of the Kimberly-Clark pulp mill in Terrace Bay due to complete closure of Long Lac Diversion, and the reduction in livelihood and alteration in way of life for local populations associated with the closure of both diversions. -

Postglacial Water Levels in the Great Lakes Region in Relation to Holocene Climate Change: Thecamoebian and Palynological Evidence
Postglacial Water Levels in the Great Lakes Region in Relation to Holocene Climate Change: Thecamoebian and Palynological Evidence by Adam Patrick Sarvis, B.Sc. A thesis submitted to the Department of Earth Sciences in partial fulfillment of the requirements for the degree of Master of Science December, 2000 Brock University St. Catharines, Ontario © Adam Patrick Sarvis, 2000 Abstract Various lake phases have developed in the upper Great Lakes in response to isostatic adjustment and changes in water supply since the retreat of the Laurentide Ice Sheet. Georgian Bay experienced a lowstand that caused a basin wide unconformity approximately 7,500 years ago that cannot be explained by geological events. Thecamoebians are shelled protozoans abundant in freshwater environments and they are generally more sensitive to changing environmental conditions than the surrounding vegetation. Thecamoebians can be used to reconstruct the paleolimnology. The abundance of thecamoebians belonging to the genus Centropyxis, which are known to tolerate slightly brackish conditions (i.e. high concentrations of ions) records highly evaporative conditions in a closed basin. During the warmer interval (9000 to 700 yBP), the Centropyxis - dominated population diminishes and is replaced by an abundant and diverse Difflugia dominate population. Historical climate records from Tobermory and Midland, Ontario were correlated with the Lake Huron water level curve. The fossil pollen record and comparison with modem analogues allowed a paleo-water budget to be calculated for Georgian Bay. Transfer function analysis of fossil pollen data from Georgian Bay records cold, dry winters similar to modem day Minneapolis, Minnesota. Drier climates around this time are also recorded in bog environments in Southem Ontario - the drying of Lake Tonawanda and inception of paludification in Willoughby Bog, for instance, dates around 7,000 years ago. -

CEN 304 SECOND EDITION Sailing Corrected to Monthly Edition No
CEN 304 SECOND EDITION Sailing Corrected to Monthly Edition No. 02/2021 Directions Detroit River Lake St. Clair St. Clair River The Canadian Hydrographic Service produces and distributes Nautical Charts, Sailing Directions, Tide and Current Tables and the Atlas of Tidal Currents of the navigable waters of Canada. These publications are available from authorized Canadian Hydrographic Service Chart Dealers. For information about these publications, please contact: Canadian Hydrographic Service Fisheries and Oceans Canada 200 Kent Street Ottawa, Ontario Canada K1A 0E6 Phone: 613-998-4931 Toll free: 1-866-546-3613 E-mail: [email protected] or visit the CHS web site for dealer location and related information at: www.charts.gc.ca Fisheries and Oceans Canada information line 1-613-993-0999 Canadian Coast Guard Search and Rescue Rescue Co-ordination Centre Trenton (Great Lakes area) 1-800-267-7270 © Her Majesty the Queen in Right of Canada, 2020. DFO/2020-2034 Catalogue No. Fs72-3/4-2020E-PDF ISBN 978-0-660-35797-3 Ottawa, 2020 (Aussi disponible en français) Cover photograph Capt. Henry Jackman, unloading in Sarnia Photo by: CHS, Rick Martin Pictograph legend Anchorage Light Pilotage Caution Lifesaving station Radio calling-in point Current Marina Wharf CEN 304 SECOND EDITION Sailing Corrected to Monthly Edition No. 02/2021 Directions Detroit River Lake St. Clair St. Clair River Second Edition 2020 Nautical Charts Protect Lives, Property and the Marine Environment Fisheries and Oceans Canada Users of this publication are requested to forward information regarding newly discovered dangers, changes in aids to navigation, the existence of new shoals or channels, printing errors, or other information that would be useful for the correction of nautical charts and hydrographic publications affecting Canadian waters to: Director General Canadian Hydrographic Service Fisheries and Oceans Canada Ottawa, Ontario Canada K1A 0E6 Table of Contents Lake Huron Preface. -

Ontario's Stream Rehabilitation Manual
Ontario’s Stream Rehabilitation Manual Written by Mark G. Heaton Rick Grillmayer And Jack G. Imhof Illustrations By Roy DeGuisti Copyright by Ontario Streams 17266 Old Main Street, Belfountain, Ontario L0N 1B0 May 2002 All Rights Reserved. Chapter 1 Introduction The Created Need Restoration, Rehabilitation or Reclamation Building on Experience Partnerships Mean Ownership Chapter 2 Perspectives What is a Watershed? Life in a Dynamic Physical Environment ….. Eco ~ System Stream Corridors The Natural Tendency of Rivers (written by Jack G. Imhof) Chapter 3 Look Before You Leap Project Planning Basics STEP 1: Focus STEP 2: Research and Reconnaissance STEP 3: Evaluation and Impact Assessment STEP 4: Goal, Objectives and Targets Chapter 4 Charting the Course to Action! STEP 5: Plan, Prepare and Consult STEP 6: Implement STEP 7: Monitor and Report Chapter 5 Learning From The Past Gathering The Information Drawing Conclusions... Chapter 6 Techniques Introduction Barrier Management Barrier Modification Fishways Soil Bioengineering (written by R. Grillmayer) Live Staking Fascines Brushlayers Brushmattress Live Cribwall Willow Posts Native Material Revetment Live Rock Revetment Habitat Improvement L.U.N.K.E.R.S. Boulder Placement Half Log Cover Instream Log Cover The Sweeper Palette Cover Cabled Log Jam Channel Rehabilitation Natural Channel Reconstruction (Under Construction) Riparian Corridor Rehabilitation (Under Construction) Woody Debris Management Low Stage Weirs (Under Construction) Wing Deflectors Bibliography Bibliography Appendices Appendix -

Description of the Niagara Quadrangle
DESCRIPTION OF THE NIAGARA QUADRANGLE. By E. M. Kindle and F. B. Taylor.a INTRODUCTION. different altitudes, but as a whole it is distinctly higher than by broad valleys opening northwestward. Across northwestern GENERAL RELATIONS. the surrounding areas and is in general bounded by well-marked Pennsylvania and southwestern New York it is abrupt and escarpments. i nearly straight and its crest is about 1000 feet higher than, and The Niagara quadrangle lies between parallels 43° and 43° In the region of the lower Great Lakes the Glaciated Plains 4 or 5 miles back from the narrow plain bordering Lake Erie. 30' and meridians 78° 30' and 79° and includes the Wilson, province is divided into the Erie, Huron, and Ontario plains From Cattaraugus Creek eastward the scarp is rather less Olcott, Tonawanda, and Lockport 15-minute quadrangles. It and the Laurentian Plateau. (See fig. 2.) The Erie plain abrupt, though higher, and is broken by deep, narrow valleys thus covers one-fourth of a square degree of the earth's sur extending well back into the plateau, so that it appears as a line face, an area, in that latitude, of 870.9 square miles, of which of northward-facing steep-sided promontories jutting out into approximately the northern third, or about 293 square miles, the Erie plain. East of Auburn it merges into the Onondaga lies in Lake Ontario. The map of the Niagara quadrangle shows escarpment. also along its west side a strip from 3 to 6 miles wide comprising The Erie plain extends along the base of the Portage escarp Niagara River and a small area in Canada. -

Great Lakes Research Review
Volume 8 2011 Great Lakes Research Review Great Lakes Research Consortium The Saint Lawrence River: Flowing towards Regional Governance Volume 8 2011 Great Lakes Research Review TABLE OF CONTENTS Introduction to The Saint Lawrence River: Flowing towards Regional Governance Michael R. Twiss, Jeffrey J. Ridal, and Martin Heintzelman .........................................4 A Sustainable Future for the Great Lakes and Saint Lawrence River Region Rick Findlay ....................................................................................................................5 Governance along the St. Lawrence River: Expanding Beyond Remedial Action Plans Alicia L. Ritcey, Linda M. Campbell, and Jeffrey J. Ridal ...........................................11 The St. Lawrence River Flowing Forward Together Remedial Action Plans for Areas of Concern and Their Role in Saint Lawrence River Governance Katherine Beehler and Jeffrey J. Ridal .........................................................................18 The St. Lawrence Seaway and Power Dam Project: Construction Feat – Economic Failure Claire Puccia Parham ....................................................................................................22 Making the Links: Pollution, Poverty, and Environmental Justice Renee Griffin and Theresa McClenaghan .....................................................................27 The Struggle to Balance Environmental and Economic Interests on the Danube River Suzanne Ebert ...............................................................................................................34