Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ni33nh3+3Nhi
Pure & Appi. Chem., Vol. 49, pp.67—73. Pergamon Press, 1977. Printed in Great Britain. NON-AQJEOUS SOLVENTS FOR PREPARATION AND REACTIONS OF NITROGEN HALOGEN COMPOUNDS Jochen Jander Department of Chemistry, University of Heidelberg, D 69 Heidelberg, Germany Abstract -Theimportance of non—aqueous solvents for the chemistry of haloamines is shown in more recent reaction examples. The solvents must be low-melting because of the general temperature sensitivity of the haloamines. They may take part in the reaction (formation of CH NI •CH NH (CH )2NI, NI-,.5 NH-, and NH0I.NH-)ormay e ner (ormation of I .quinuiilidin, 2 NI •1 trithylenediamine, [I(qunuclidine),] salts pure NBr, and compounds of the type R1R2NC103 with R1 and R2 =orgnicgroup or hydrogen). INTRODUCTION Non-aqueous solvents play an important role in preparation and reactions of nitrogen halogen compounds. Due to the fact, that all haloamines are derivatives of ammonia or amines, liquid ammonia or liquid organic amines are used as solvents very often. Liquid ammonia and the lower organic amines reveal, in addition, the advantage of low melting points (liquid ammonia -78, liquid monomethylamine -93.5, liquid dimethylamine -92, liquid tn-. methylamine -12k°C); these meet the general temperature sensitivity of the nitrogen halogen compounds, especially of the pure .lorganic ones. Ammonia and amines are very seldom inert solvents; they mostly take part in the reaction planned for synthesis or conversion of the haloamine. In case a reaction of the solvent is to be avoided, one has to look for a low melting inert solvent, for example methylene chloride (m.p. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

2019 Sodium Iodide
VETPAK SAFETY DATA SHEET Section 1: Identification of the Substance or Mixture and of the Supplier Product Name: Sodium Iodide. Other Names: Sodium monoiodide. Recommended Use: Reagent in analytical chemistry, Photographic emulsions (precipitating silver), Feed additive, Spectroscopy, Infrared transmission, dietary supplement (up to 0.01% in table salt). Company Details: Vetpak Ltd. Address: 249 Bruce Berquist Dr, Te Awamutu 3800. Telephone Number: (07) 870 2024 Emergency Telephone Number: (0800) 764-766 24 hours. National Poisons Centre, Department of Preventative and Social Medicine, University of Otago, P O Box 913, Dunedin, New Zealand. (07) 870 2024 Vetpak. 8.00am to 5.00pm Monday to Friday except public holidays. Date of Preparation: 31st July 2019 Section 2: Hazards Identification STATEMENT OF HAZARDOUS NATURE This product is HAZARDOUS IN THIS FORM AND AT THIS STRENGTH. Handle correctly and as directed by this SDS. EPA New Zealand approval code: HSR003606 HAZARD LABELLING WARNING HAZARD CLASSIFICATION AND STATEMENTS HSNO HSNO GHS Signal Word GHS Hazard Statement 6.1E Acute Toxicity (All) Category 5 Warning H303 H313 H333 May be harmful if swallowed, in contact with the skin, inhaled 6.5B Contact sensitiser Category 1 Warning H317 May cause an allergic skin reaction 6.9A Harmful to human target Category 1 Danger H370 H372 Causes damage organs or systems to organs 9.1A Aquatic toxicity Category 1 Warning H400 Very toxic to aquatic life Prevention Statements: P102: Keep out of reach of children. P103: Read label before use. P260: Do not breathe mist/vapours/spray P261: Avoid breathing dust P264: Wash hands thoroughly after use. P270: Do not eat, drink or smoke when handling this product. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

IGNITION! an Informal History of Liquid Rocket Propellants by John D
IGNITION! U.S. Navy photo This is what a test firing should look like. Note the mach diamonds in the ex haust stream. U.S. Navy photo And this is what it may look like if something goes wrong. The same test cell, or its remains, is shown. IGNITION! An Informal History of Liquid Rocket Propellants by John D. Clark Those who cannot remember the past are condemned to repeat it. George Santayana RUTGERS UNIVERSITY PRESS IS New Brunswick, New Jersey Copyright © 1972 by Rutgers University, the State University of New Jersey Library of Congress Catalog Card Number: 72-185390 ISBN: 0-8135-0725-1 Manufactured in the United Suites of America by Quinn & Boden Company, Inc., Rithway, New Jersey This book is dedicated to my wife Inga, who heckled me into writing it with such wifely re marks as, "You talk a hell of a fine history. Now set yourself down in front of the typewriter — and write the damned thing!" In Re John D. Clark by Isaac Asimov I first met John in 1942 when I came to Philadelphia to live. Oh, I had known of him before. Back in 1937, he had published a pair of science fiction shorts, "Minus Planet" and "Space Blister," which had hit me right between the eyes. The first one, in particular, was the earliest science fiction story I know of which dealt with "anti-matter" in realistic fashion. Apparently, John was satisfied with that pair and didn't write any more s.f., kindly leaving room for lesser lights like myself. -
Safety Data Sheet
Safety Data Sheet Classified According to OSHA Hazard Communication Standard (HCS) SECTION 1: Identification 1.1. Product Identifier Trade Name or Designation: Potassium Iodide, ACS Reagent Grade Product Number: RDCP0560 Other Identifying Product Numbers: RDCP0560-100B1, RDCP0560-12F4, RDCP0560-250A6, RDCP0560-3A5, RDCP0560-500B1, RDCP0560-5C1 1.2. Recommended Use and Restrictions on Use General Laboratory Reagent 1.3. Details of the Supplier of the Safety Data Sheet Company: Ricca Chemical Company Address: 448 West Fork Drive Arlington, TX 76012 USA Telephone: 888-467-4222 1.4. Emergency Telephone Number (24 hours) CHEMTREC (USA) 800-424-9300 CHEMTREC (International) 1+ 703-527-3887 SECTION 2: Hazard(s) Identification 2.1. Classification of the Substance or Mixture For the full text of the Hazard and Precautionary Statements listed below, see Section 16. Hazard Hazard Class Category Statements Precautionary Statements: Eye Damage / Irritation Category 2B H320 P264, P305+P351+P338, P337+P313 Skin Sensitizer Category 1 H317 P261, P272, P280, P302+P352, P332+P313, P321, P363, P501 Reproductive Toxicity Category 2 H361 P201, P202, P280, P308+P313, P405, P501 Specific Target Organs/Systemic Toxicity Following Repeated Category 1 H372 P260, P264, P270, P314, P501 Exposure Hazardous to the Aquatic Environment (Chronic) Category 2 H411 P273, P391, P501 Product Number: RDCP0560 Page 1 of 12 Safety Data Sheet 2.2. GHS Label Elements Pictograms: Signal Word: Danger Hazard Statements: Hazard Number Hazard Statement H317 May cause an allergic skin reaction. H320 Causes eye irritation. H361 Suspected of damaging fertility or the unborn child. H372 Causes damage to organs through prolonged or repeated exposure. -

"Perchloric Acid and Perchlorates". In: Kirk-Othmer Encyclopedia Of
PERCHLORIC ACID AND PERCHLORATES 1. Introduction Perchloric acid [7601-90-3], HClO4, is one of the strongest of the mineral acids. The perchlorates are more stable than the other chlorine oxyanions, ie, chlorates, À À À ClO 3; chlorites, ClO 2; or hypochlorites, OCl (3) (see DICHLORINE MONOXIDE, HYPOCHLOROUS ACID, AND HYPOCHLORITES). Essentially, all of the commercial perchlo- rate compounds are prepared either directly or indirectly by electrochemical oxi- dation of chlorine compounds (4–8) (see CHLORINE; ELECTROCHEMICAL PROCESSING, INTRODUCTION). The perchlorates of practically all the electropositive metals are known, except for a few cations having low charges. The most outstanding property of the perchlorates is their oxidizing ability. On heating, these compounds decompose into chlorine, chlorides, and oxygen gas. Aqueous perchlorate solutions exhibit little or no oxidizing power when dilute or cold. However, hot concentrated perchloric acid is a powerful oxidizer and whenever it contacts oxidizable matter extreme caution is required. The acidified concentrated solutions of perchlorate salts must also be handled with caution. Ammonium perchlorate [7790-98-9] (AP) is one of the most important perchlorates owing to its high (54.5%) O2 content and the absence of residue on decomposition. These properties, along with a long shelf life, make it a useful rocket propellant (see EXPLOSIVES AND PROPELLANTS) (9). AP is a true explosive as demonstrated by the explosion at the PEPCON plant at Henderson, Nevada, in 1988 (10). Following early (1890s) work (11), France, Germany, Switzerland, and the United States began to produce perchlorates for use as propellants and explo- sives. Whereas total world production of perchlorates did not exceed 1800 t/yr until 1940, it increased dramatically during World War II to about 18,000 t/yr in order to supply the rocket and missile industries. -

Bnl Chemical Storage Table
The only official copy of this document is on-line at the SBMS website. Before using a printed copy, verify that it is current by checking the effective date. BNL CHEMICAL STORAGE RECOMMENDATIONS This information is adapted from published references for the most common chemicals at BNL. It presents well known compatibility and storage issues but is not an inclusive list of chemicals, hazards, or compatibility issues. For guidance on chemicals not listed on this table, consult the Material Safety Data Sheet, the BNL Chemical Hygiene Officer or your Environmental Safety & Health Representative. The information provided in these tables is to serve as guidance for common work settings and may be modified as appropriate based on the quantity, container type, storage containment & safety apparatus, and work conditions. BNL CHEMICAL STORAGE INFORMATION FOR TABLE 1: COMPRESSED GASES IN CYLINDERS RECOMMENDED COLOR-CODING SYSTEM TABLE 2A: FOR SOLIDS AND LIQUIDS CONTAINER STORAGE BNL CHEMICAL STORAGE INFORMATION FOR TABLE 2B: CONTAINERS OF SOLIDS AND LIQUIDS 12.0/12029e011.pdf 1 of 22 (02/2011) The only official copy of this document is on-line at the SBMS website. Before using a printed copy, verify that it is current by checking the effective date. TABLE 1: BNL CHEMICAL STORAGE INFORMATION FOR COMPRESSED GASES IN CYLINDERS Note: See the Compressed Gas Cylinders and Related Systems Subject Area for additional information and requirements. Global Type Examples Harmonization Storage Symbol Can be stored with all CORROSIVE other classes unless the -

Material Safety Data Sheet Potassium Iodide Solutions, 5% and 10%
Page 1 of 5 Material Safety Data Sheet Potassium Iodide Solutions, 5% and 10% ACC# 40153 Section 1 - Chemical Product and Company Identification MSDS Name: Potassium Iodide Solutions, 5% and 10% Catalog Numbers: SP241-1, SP242-1, SP242-4, SP242-500 Synonyms: None Company Identification: Fisher Scientific 1 Reagent Lane Fair Lawn, NJ 07410 For information, call: 201-796-7100 Emergency Number: 201-796-7100 For CHEMTREC assistance, call: 800-424-9300 For International CHEMTREC assistance, call: 703-527-3887 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 7732-18-5 Water 90-95 231-791-2 7681-11-0 POTASSIUM IODIDE 5-10 231-659-4 Hazard Symbols: None listed. Risk Phrases: None listed. Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: clear, colorless liquid. Caution! May cause respiratory tract irritation. May cause skin irritation. May cause eye irritation. This substance has caused adverse reproductive and fetal effects in animals. Causes digestive tract irritation. Target Organs: Thyroid. Potential Health Effects Eye: Causes eye irritation. Skin: May cause skin irritation. Chronic ingestion of iodides during pregnancy has resulted in fetal death, severe goiter, and cretinoid appearance of the newborn. Ingestion: Causes gastrointestinal irritation with nausea, vomiting and diarrhea. Inhalation: May cause respiratory tract irritation. Chronic: Chronic exposure can lead to iodism characterized by salivation, nasal discharge, sneezing, conjunctivitis, fever, laryngitis, bronchitis, stomatitis, and skin rashes. Chronic ingestion of iodides during pregnancy has resulted in fetal death, severe goiter, and cretinoid appearance of the newborn. Section 4 - First Aid Measures https://fscimage.fishersci.com/msds/40153.htm 11/15/2004 Page 2 of 5 Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. -

MSDS Iodine Potassium Iodide Solution
He a lt h 1 0 Fire 0 1 0 Re a c t iv it y 0 Pe rs o n a l Pro t e c t io n J Material Safety Data Sheet Iodine Potassium Iodide TS MSDS Section 1: Chemical Product and Company Identification Product Name: Iodine Potassium Iodide TS Contact Information: Catalog Codes: SLI1021 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: Mixture. Houston, Texas 77396 RTECS: Not applicable. US Sales: 1-800-901-7247 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Water; Iodine; Potassium Iodide Order Online: ScienceLab.com CI#: Not available. CHEMTREC (24HR Emergency Telephone), call: 1-800-424-9300 Synonym: Iodine Potassium Iodide TS International CHEMTREC, call: 1-703-527-3887 Chemical Name: Not applicable. For non-emergency assistance, call: 1-281-441-4400 Chemical Formula: Not applicable. Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Water 7732-18-5 92 Iodine 7553-56-2 2 Potassium Iodide 7681-11-0 6 Toxicological Data on Ingredients: Iodine: ORAL (LD50): Acute: 14000 mg/kg [Rat]. 22000 mg/kg [Mouse]. Potassium Iodide LD50: Not available. LC50: Not available. Section 3: Hazards Identification Potential Acute Health Effects: Slightly hazardous in case of skin contact (corrosive, irritant), of eye contact (irritant, corrosive), of ingestion. Potential Chronic Health Effects: CARCINOGENIC EFFECTS: Not available. MUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. [Potassium Iodide]. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/ female, Development toxin [POSSIBLE] [Potassium Iodide]. The substance may be toxic to blood, skin, eyes, thyroid. Repeated or prolonged exposure to the substance can produce target organs damage. -
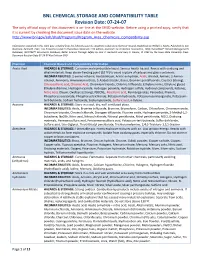
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers -

Chlorine Oxides and Chlorine Oxygen Acids 1
Chlorine Oxides and Chlorine Oxygen Acids 1 Chlorine Oxides and Chlorine Oxygen Acids Helmut Vogt, Technische Fachhochschule Berlin, Berlin, Federal Republic of Germany (Chaps. 1, 4.2 – 4.6, 7, and 9) Jan Balej, Ingenieurburo¨ fur¨ chemische Technik, Julich,¨ Federal Republic of Germany (Chap. 1) John E. Bennett, Eltech Systems Corp., Fairport Harbor, Ohio 44077, United States (Chaps. 4.2 – 4.6) Peter Wintzer, Cellulosefabrik Attisholz AG, Luterbach, Switzerland (Chaps. 7 and 9) Saeed Akbar Sheikh, Davy McKee AG, Frankfurt, Federal Republic of Germany (Chaps. 2, 3, 4.1, 5, 6, and 9) Patrizio Gallone, Politecnico di Milano, Milano, Italy (Chaps. 8 and 9) 1. Introduction ............... 2 7. Chloric Acid and Chlorates ..... 21 2. Hypochlorous Acid ........... 5 7.1. Properties ................. 22 3. Solid Hypochlorites ........... 5 7.2. Production Fundamentals ...... 24 3.1. Properties ................. 5 7.2.1. Chlorate-Generating Reactions .... 24 3.2. Production ................ 6 7.2.2. Loss Reactions .............. 26 3.3. Quality Specifications ......... 7 7.3. Industrial Electrosynthesis Systems 27 3.4. Uses ..................... 7 7.3.1. Electrolysis Cell Types ......... 28 4. Hypochlorite Solutions ........ 7 7.3.2. Electrodes ................. 28 4.1. Chemical Production .......... 7 7.3.3. Operational Parameters ......... 31 ............... 4.1.1. From Chlorine 8 7.3.4. Brine Purification ............. 33 ........ 4.1.2. From Bleaching Powder 8 7.4. Crystallization .............. 33 ............. 4.2. Electrosynthesis 9 7.5. Construction Materials ........ 33 ......... 4.2.1. Reaction Fundamentals 9 7.6. Environmental Protection ...... 34 .............. 4.2.2. Industrial Cells 10 7.7. Quality Specifications ......... 34 ................... 4.3. Storage 14 7.8. Storage, Transportation, and Safety 34 4.4.