Quantitative Paleontology for Mathematica User's Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Download PDF File
1.08 1.19 1.46 Nimravus brachyops Nandinia binotata Neofelis nebulosa 115 Panthera onca 111 114 Panthera atrox 113 Uncia uncia 116 Panthera leo 112 Panthera pardus Panthera tigris Lynx issiodorensis 220 Lynx rufus 221 Lynx pardinus 222 223 Lynx canadensis Lynx lynx 119 Acinonyx jubatus 110 225 226 Puma concolor Puma yagouaroundi 224 Felis nigripes 228 Felis chaus 229 Felis margarita 118 330 227 331Felis catus Felis silvestris 332 Otocolobus manul Prionailurus bengalensis Felis rexroadensis 99 117 334 335 Leopardus pardalis 44 333 Leopardus wiedii 336 Leopardus geoffroyi Leopardus tigrinus 337 Pardofelis marmorata Pardofelis temminckii 440 Pseudaelurus intrepidus Pseudaelurus stouti 88 339 Nimravides pedionomus 442 443 Nimravides galiani 22 338 441 Nimravides thinobates Pseudaelurus marshi Pseudaelurus validus 446 Machairodus alberdiae 77 Machairodus coloradensis 445 Homotherium serum 447 444 448 Smilodon fatalis Smilodon gracilis 66 Pseudaelurus quadridentatus Barbourofelis morrisi 449 Barbourofelis whitfordi 550 551 Barbourofelis fricki Barbourofelis loveorum Stenogale Hemigalus derbyanus 554 555 Arctictis binturong 55 Paradoxurus hermaphroditus Genetta victoriae 553 558 Genetta maculata 559 557 660 Genetta genetta Genetta servalina Poiana richardsonii 556 Civettictis civetta 662 Viverra tangalunga 661 663 552 Viverra zibetha Viverricula indica Crocuta crocuta 666 667 Hyaena brunnea 665 Hyaena hyaena Proteles cristata Fossa fossana 664 669 770 Cryptoprocta ferox Salanoia concolor 668 772 Crossarchus alexandri 33 Suricata suricatta 775 -

PHYLOGENETIC SYSTEMATICS of the BOROPHAGINAE (CARNIVORA: CANIDAE) Xiaoming Wang
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Mammalogy Papers: University of Nebraska State Museum, University of Nebraska State Museum 1999 PHYLOGENETIC SYSTEMATICS OF THE BOROPHAGINAE (CARNIVORA: CANIDAE) Xiaoming Wang Richard H. Tedford Beryl E. Taylor Follow this and additional works at: http://digitalcommons.unl.edu/museummammalogy This Article is brought to you for free and open access by the Museum, University of Nebraska State at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Mammalogy Papers: University of Nebraska State Museum by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. PHYLOGENETIC SYSTEMATICS OF THE BOROPHAGINAE (CARNIVORA: CANIDAE) XIAOMING WANG Research Associate, Division of Paleontology American Museum of Natural History and Department of Biology, Long Island University, C. W. Post Campus, 720 Northern Blvd., Brookville, New York 11548 -1300 RICHARD H. TEDFORD Curator, Division of Paleontology American Museum of Natural History BERYL E. TAYLOR Curator Emeritus, Division of Paleontology American Museum of Natural History BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 243, 391 pages, 147 figures, 2 tables, 3 appendices Issued November 17, 1999 Price: $32.00 a copy Copyright O American Museum of Natural History 1999 ISSN 0003-0090 AMNH BULLETIN Monday Oct 04 02:19 PM 1999 amnb 99111 Mp 2 Allen Press • DTPro System File # 01acc (large individual, composite figure, based Epicyon haydeni ´n. (small individual, based on AMNH 8305) and Epicyon saevus Reconstruction of on specimens from JackNorth Swayze America. Quarry). Illustration These by two Mauricio species Anto co-occur extensively during the late Clarendonian and early Hemphillian of western 2 1999 WANG ET AL.: SYSTEMATICS OF BOROPHAGINAE 3 CONTENTS Abstract .................................................................... -

Bayesian Phylogenetic Estimation of Fossil Ages Rstb.Royalsocietypublishing.Org Alexei J
Downloaded from http://rstb.royalsocietypublishing.org/ on June 20, 2016 Bayesian phylogenetic estimation of fossil ages rstb.royalsocietypublishing.org Alexei J. Drummond1,2 and Tanja Stadler2,3 1Department of Computer Science, University of Auckland, Auckland 1010, New Zealand 2Department of Biosystems Science and Engineering, Eidgeno¨ssische Technische Hochschule Zu¨rich, 4058 Basel, Switzerland Research 3Swiss Institute of Bioinformatics (SIB), Lausanne, Switzerland Cite this article: Drummond AJ, Stadler T. Recent advances have allowed for both morphological fossil evidence 2016 Bayesian phylogenetic estimation of and molecular sequences to be integrated into a single combined inference fossil ages. Phil. Trans. R. Soc. B 371: of divergence dates under the rule of Bayesian probability. In particular, 20150129. the fossilized birth–death tree prior and the Lewis-Mk model of discrete morphological evolution allow for the estimation of both divergence times http://dx.doi.org/10.1098/rstb.2015.0129 and phylogenetic relationships between fossil and extant taxa. We exploit this statistical framework to investigate the internal consistency of these Accepted: 3 May 2016 models by producing phylogenetic estimates of the age of each fossil in turn, within two rich and well-characterized datasets of fossil and extant species (penguins and canids). We find that the estimation accuracy of One contribution of 15 to a discussion meeting fossil ages is generally high with credible intervals seldom excluding the issue ‘Dating species divergences using rocks true age and median relative error in the two datasets of 5.7% and 13.2%, and clocks’. respectively. The median relative standard error (RSD) was 9.2% and 7.2%, respectively, suggesting good precision, although with some outliers. -

2014BOYDANDWELSH.Pdf
Proceedings of the 10th Conference on Fossil Resources Rapid City, SD May 2014 Dakoterra Vol. 6:124–147 ARTICLE DESCRIPTION OF AN EARLIEST ORELLAN FAUNA FROM BADLANDS NATIONAL PARK, INTERIOR, SOUTH DAKOTA AND IMPLICATIONS FOR THE STRATIGRAPHIC POSITION OF THE BLOOM BASIN LIMESTONE BED CLINT A. BOYD1 AND ED WELSH2 1Department of Geology and Geologic Engineering, South Dakota School of Mines and Technology, Rapid City, South Dakota 57701 U.S.A., [email protected]; 2Division of Resource Management, Badlands National Park, Interior, South Dakota 57750 U.S.A., [email protected] ABSTRACT—Three new vertebrate localities are reported from within the Bloom Basin of the North Unit of Badlands National Park, Interior, South Dakota. These sites were discovered during paleontological surveys and monitoring of the park’s boundary fence construction activities. This report focuses on a new fauna recovered from one of these localities (BADL-LOC-0293) that is designated the Bloom Basin local fauna. This locality is situated approximately three meters below the Bloom Basin limestone bed, a geographically restricted strati- graphic unit only present within the Bloom Basin. Previous researchers have placed the Bloom Basin limestone bed at the contact between the Chadron and Brule formations. Given the unconformity known to occur between these formations in South Dakota, the recovery of a Chadronian (Late Eocene) fauna was expected from this locality. However, detailed collection and examination of fossils from BADL-LOC-0293 reveals an abundance of specimens referable to the characteristic Orellan taxa Hypertragulus calcaratus and Leptomeryx evansi. This fauna also includes new records for the taxa Adjidaumo lophatus and Brachygaulus, a biostratigraphic verifica- tion for the biochronologically ambiguous taxon Megaleptictis, and the possible presence of new leporid and hypertragulid taxa. -

Genus/Species Skull Ht Lt Wt Stage Range Abacinonyx See Acinonyx Abathomodon See Speothos A
Genus/Species Skull Ht Lt Wt Stage Range Abacinonyx see Acinonyx Abathomodon see Speothos A. fossilis see Icticyon pacivorus? Pleistocene Brazil Abelia U.Miocene Europe Absonodaphoenus see Pseudarctos L.Miocene USA A. bathygenus see Cynelos caroniavorus Acarictis L.Eocene W USA cf. A. ryani Wasatchian Colorado(US) A. ryani Wasatchian Wyoming, Colorado(US) Acinomyx see Acinonyx Acinonyx M.Pliocene-Recent Europe,Asia,Africa,N America A. aicha 2.3 m U.Pliocene Morocco A. brachygnathus Pliocene India A. expectata see Miracinonyx expectatus? Or Felis expectata? A. intermedius M.Pleistocene A. jubatus living Cheetah M.Pliocene-Recent Algeria,Europe,India,China A. pardinensis 91 cm 3 m 60 kg Astian-Biharian Italy,India,China,Germany,France A. sp. L.Pleistocene Tanzania,Ethiopia A. sp. Cf. Inexpectatus Blancan-Irvingtonian California(US) A. studeri see Miracinonyx studeri Blancan Texas(US) A. trumani see Miracinonyx trumani Rancholabrean Wyoming,Nevada(US) Acionyx possibly Acinonyx? A. cf. Crassidens Hadar(Ethiopia) Acrophoca 1.5 m U.Miocene-L.Pliocene Peru,Chile A. longirostris U.Miocene-L.Pliocene Peru A. sp. U.Miocene-L.Pliocene Chile Actiocyon M-U.Miocene W USA A. leardi Clarendonian California(US) A. sp. M.Miocene Oregon(US) Adcrocuta 82 cm 1.5 m U.Miocene Europe,Asia A. advena A. eximia 80 cm 1.5 m Vallesian-Turolian Europe(widespread),Asia(widespread) Adelphailurus U.Miocene-L.Pliocene W USA, Mexico,Europe A. kansensis Hemphillian Arizona,Kansas(US),Chihuahua(Mexico) Adelpharctos M.Oligocene Europe Adilophontes L.Miocene W USA A. brachykolos Arikareean Wyoming(US) Adracodon probably Adracon Eocene France A. quercyi probably Adracon quercyi Eocene France Adracon U.Eocene-L.Oligocene France A. -

1: Beast2.1.3 R1
1: Beast2.1.3_r1 0.93 Canis adustus 0.89 Canis mesomelas 0.37 Canis simensis Canis arnensis 1 Lycaon pictus 1 Cuon javanicus 0.57 Xenocyon texanus 0.91 Canis antonii 0.96 0.35 Canis falconeri 1 Canis dirus 1 Canis armbrusteri 0.060.27 Canis lupus Canis chihliensis Canis etruscus 0.98 0.99 0.26 Canis variabilis 0.11 Canis edwardii 0.93 Canis mosbachensis 0.68 0.58 Canis latrans Canis aureus 0.94 Canis palmidens 0.94 Canis lepophagus 1 0.41 Canis thooides Canis ferox 0.99 Eucyon davisi 0.8 Cerdocyon texanus Vulpes stenognathus Leptocyon matthewi 0.98 Urocyon cinereoargenteus 0.85 Urocyon minicephalus 0.96 1 Urocyon citronus 1 Urocyon galushi 0.96 Urocyon webbi 1 1 Metalopex merriami 0.23 Metalopex macconnelli 0.68 Leptocyon vafer 1 Leptocyon leidyi Leptocyon vulpinus 0.7 Leptocyon gregorii 1 Leptocyon douglassi Leptocyon mollis 0.32 Borophagus dudleyi 1 Borophagus hilli 0.98 Borophagus diversidens 0.97 Borophagus secundus 0.18 Borophagus parvus 1 Borophagus pugnator 1 Borophagus orc 1 Borophagus littoralis 0.96 Epicyon haydeni 1 0.92 Epicyon saevus Epicyon aelurodontoides Protepicyon raki 0.98 0.28 Carpocyon limosus 0.96 Carpocyon webbi 0.97 0.98 Carpocyon robustus Carpocyon compressus 0.17 0.87 Paratomarctus euthos Paratomarctus temerarius Tomarctus brevirostris 0.31 0.85 0.35 Tomarctus hippophaga 0.17 Tephrocyon rurestris Protomarctus opatus 0.24 0.58 Microtomarctus conferta Metatomarctus canavus Psalidocyon marianae 0.79 1 Aelurodon taxoides 0.32 0.27 Aelurodon ferox 1 Aelurodon stirtoni 0.14 Aelurodon mcgrewi 0.33 Aelurodon asthenostylus -
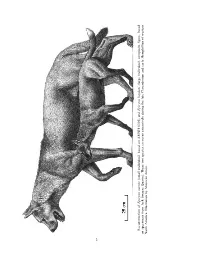
File # 01Acc (Large Individual, Composite figure, Based Epicyon Haydeni «N
AMNH BULLETIN Monday Oct 04 02:19 PM 1999 amnb 99111 Mp 2 Allen Press • DTPro System File # 01acc (large individual, composite figure, based Epicyon haydeni ´n. (small individual, based on AMNH 8305) and Epicyon saevus Reconstruction of on specimens from JackNorth Swayze America. Quarry). Illustration These by two Mauricio species Anto co-occur extensively during the late Clarendonian and early Hemphillian of western 2 1999 WANG ET AL.: SYSTEMATICS OF BOROPHAGINAE 3 CONTENTS Abstract ..................................................................... 9 Introduction .................................................................. 10 Institutional Abbreviations ................................................... 11 Acknowledgments .......................................................... 12 History of Study .............................................................. 13 Materials and Methods ........................................................ 18 Scope ...................................................................... 18 Species Determination ........................................................ 18 Taxonomic Nomenclature ..................................................... 19 Format ..................................................................... 19 Chronological Framework .................................................... 20 Definitions .................................................................. 21 Systematic Paleontology ....................................................... 21 Subfamily Borophaginae Simpson, -

A Review of Selected Features of the Family Canidae with Reference to Its Fundamental Taxonomic Status Barnabas Pendragon
Papers A review of selected features of the family Canidae with reference to its fundamental taxonomic status Barnabas Pendragon Dogs comprise 35 extant species in 14 genera. They belong to the order Carnivora, which has common morphological and karyotypic features. Within the order, member species can be grouped based on heterologous DNA melting temperatures. The family Canidae forms such a group. Selected features of the Canidae are reviewed here in order to examine the fundamental taxonomic status of this family. Hybrid relationships demonstrate that the family Canidae is a single, reproductively compatible group having the taxonomic status of basic type. As opposed to the various species in the family whose formation was accompanied by genetic change, establishment of the domestic dog was accompanied by almost no genetic change; genetically all domestic dogs are grey wolves. The remarkable variation observed among the various breeds of domestic dog reflects the potential for morphological change hard-wired into the canid genome. The basic type appears to be divided into two subfamilies in the Cenozoic strata; the extant Caninae and the extinct Borophaginae. The ‘oldest’ known canid species is Prohesperocyon, which is found in upper Eocene fossil deposits. Q*HRUJH9HVWD0LVVRXULODZ\HUJDYHXVWKHDGDJH for slicing muscle tissue. However, in the omnivorous I“A dog is a man’s best friend”. More so than any other carnivores such as the bears, true carnassial teeth do not animal, dogs represent friendship and companionship. develop. Nevertheless, dogs, the Canidae, are beasts of prey Interestingly, the carnivore order has a largely belonging to the order Carnivora. They have long slender conservative Karyotype. -

Paleoecology of Fossil Species of Canids (Canidae, Carnivora, Mammalia)
UNIVERSITY OF SOUTH BOHEMIA, FACULTY OF SCIENCES Paleoecology of fossil species of canids (Canidae, Carnivora, Mammalia) Master thesis Bc. Isabela Ok řinová Supervisor: RNDr. V ěra Pavelková, Ph.D. Consultant: prof. RNDr. Jan Zrzavý, CSc. České Bud ějovice 2013 Ok řinová, I., 2013: Paleoecology of fossil species of canids (Carnivora, Mammalia). Master thesis, in English, 53 p., Faculty of Sciences, University of South Bohemia, České Bud ějovice, Czech Republic. Annotation: There were reconstructed phylogeny of recent and fossil species of subfamily Caninae in this study. Resulting phylogeny was used for examining possible causes of cooperative behaviour in Caninae. The study tried tu explain evolution of social behavior in canids. Declaration: I hereby declare that I have worked on my master thesis independently and used only the sources listed in the bibliography. I hereby declare that, in accordance with Article 47b of Act No. 111/1998 in the valid wording, I agree with the publication of my master thesis in electronic form in publicly accessible part of the STAG database operated by the University of South Bohemia accessible through its web pages. Further, I agree to the electronic publication of the comments of my supervisor and thesis opponents and the record of the proceedings and results of the thesis defence in accordance with aforementioned Act No. 111/1998. I also agree to the comparison of the text of my thesis with the Theses.cz thesis database operated by the National Registry of University Theses and a plagiarism detection system. In České Bud ějovice, 13. December 2013 Bc. Isabela Ok řinová Acknowledgements: First of all, I would like to thank my supervisor Věra Pavelková for her great support, patience and many valuable comments and advice. -

UNIVERSITY of CALIFORNIA Los Angeles Carnivory
UNIVERSITY OF CALIFORNIA Los Angeles Carnivory in the Oligo-Miocene: Resource Specialization, Competition, and Coexistence Among North American Fossil Canids A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Biology by Mairin Francesca Aragones Balisi 2018 © Copyright by Mairin Francesca Aragones Balisi 2018 ABSTRACT OF THE DISSERTATION Carnivory in the Oligo-Miocene: Resource Specialization, Competition, and Coexistence Among North American Fossil Canids by Mairin Francesca Aragones Balisi Doctor of Philosophy in Biology University of California, Los Angeles 2018 Professor Blaire Van Valkenburgh, Chair Competition likely shaped the evolution of the mammalian family Canidae (dogs and their ancestors; order Carnivora). From their origin 40 million years ago (Ma), early canids lived alongside potential competitors that may have monopolized the large-carnivore niche—such as bears, bear-dogs, and nimravid saber-toothed cats—before becoming large and hypercarnivorous (diet of >70% meat) later in their history. This ecomorphological context, along with high phylogenetic resolution and an outstanding fossil record, makes North American fossil canids an ideal system for investigating how biotic interactions (e.g. competition) and ecological specialization (e.g. hypercarnivory) might influence clade evolution. Chapter 1 investigates whether ecological generalization enables species to have longer durations and broader geographic range. I developed and applied a carnivory index to 100+ ii species, processing 3708 occurrences through a new duration-estimation method accounting for varying fossil preservation. A non-linear relationship between duration and carnivory emerged: both hyper- and hypocarnivores have shorter durations than mesocarnivores. Chapter 2 tests the cost of hypercarnivory, quantified as elevated extinction risk. -

1 Supplemental Material for Matzke and Wright (2016), “Inferring Node
Supplemental Material for Matzke and Wright (2016), “Inferring node dates from tip dates in fossil Canidae: the importance of tree priors” Supplemental Introduction Terminology. The methods we refer to as “tip-dating” methods are often called “total evidence” dating methods. As originally devised, these methods combined molecular and morphological data with tip-dates. However, in this study, we use a morphology-only dataset, but the models and methods are otherwise the same. So, we refer to the methods as “tip-dating” rather than “total evidence” in this paper. Brief review of tip-dating studies. Major papers have introduced tip-dating methods and models [1-6]. A number of tip-dating studies have been published at the time of writing [4, 5, 7-23]. A review of this literature, while generally approving, shows that some studies consider some of their tip-dating results implausible (e.g. [9, 22, 24]), and some infer dates that are wildly uncertain [12, 17, 25]. Evaluation of the methods against each other, or against expectations based on the fossil record, is hampered by the complexity of Bayesian analyses: differences in results might be produced by differences in clock models, tree models, site models, priors (user- set or default) on any of the parameters used in these models, issues in implementation (bugs in the code, decisions about defaults, MCMC operators, etc.), user error in setting up the analysis or post-analysis processing, and/or issues with the data itself. Canidae background. The bulk of canid evolution occurred in North America from the Eocene to present, and their fossil record is approximately continuous, with fossil diversity greater than extant diversity (approximately 35 living species, at least 123 well-described fossil taxa). -

Proportion of Species Richness 50 Borophagine 45 Canine 40 3 35 30 19 31 25 41
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title The Importance of Phylogeny in Regional and Temporal Diversity and Disparity Dynamics Permalink https://escholarship.org/uc/item/1cp9v9rh Author Ferrer, Elizabeth Anne Publication Date 2015 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California The Importance of Phylogeny in Regional and Temporal Diversity and Disparity Dynamics By Elizabeth Anne Ferrer A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Kevin Padian, Chair Professor Charles Marshall Professor Jun Sunseri Summer 2015 Abstract The Importance of Phylogeny in Regional and Temporal Diversity and Disparity Dynamics By Elizabeth Anne Ferrer Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Kevin Padian, Chair To understand how various patterns of biodiversity evolve, we must understand not only how various factors influence these patterns, but also the effects of evolutionary history on these patterns. A continuing discussion in biology is the relationship among various levels and forms of diversity. Most studies that focus on past, current, or predicted future changes in diversity use a phylogenetic context, yet lack a phylogenetic framework. Closing that conceptual gap can help to produce a more coherent understanding of diversity patterns and can be more useful when integrated with new dimensions (i.e., time). Here I focus on how extinction and origination rates affect measures of taxonomic (taxic), phylogenetic (sensu Faith’s diversity), and morphological diversity. In chapter 1, I analyze the relationship between taxonomic and phylogenetic diversity in canids and varanids using time calibrated phylogenies.