Weighing the Trade-Offs of a Direct Presence in Japan's Rare And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2006 2012 2013 2014 Introducing HALO® 2 Fused-Core®
Introducing HALO® 2 Fused-Core® UHPLC Columns From Advanced Materials Technology Rugged ∙ High Efficiency ∙ Low Back Pressure HALO 2 Fused-Core particles are designed to address the disadvantages inherent in existing sub-2 micron non-core UHPLC columns. HALO 2 UHPLC columns have all of the advantages of sub-2 µm non-core particle columns and will deliver 300,000 plates per meter efficiency (higher than existing non-core sub-2 µm columns). Manufactured with 1.0 µm frits on the column inlet, HALO 2 columns are less susceptible to column plugging. These columns can be used up to 1,000 bar (14,500 psi), but will actually produce ~20% lower back pressure than most commercially available sub-2 µm UHPLC columns under the same conditions. 1 Advantages of HALO 2 Fused-Core Columns vs. Sub-2 µm Non-core UHPLC Columns · Fused-Core UHPLC columns with ~300K plates per meter · All of the advantages of sub-2 µm non-core particles at lower - Higher efficiency than existing non-core sub-2 µm columns operating pressures · Longer column lifetime – more injections, less downtime - High speed and efficiency with short columns - Due to Fused-Core 2 micron particle architecture, 1 micron - Improved productivity from faster analyses frits can be used on the column inlet - Less solvent usage from shorter analysis times - 1 micron frits are less likely to be plugged by UHPLC samples - High resolution and peak capacity in longer columns or mobile phase contaminants than typical 0.2 – 0.5 µm - Sharper, taller peaks = better sensitivity and lower LOD frits on sub-2 -

Game Enforcer Is Just a Group of People Providing You with Information and Telling You About the Latest Games
magazine you will see the coolest ads and Letter from The the most legit info articles you can ever find. Some of the ads include Xbox 360 skins Editor allowing you to customize your precious baby. Another ad is that there is an amazing Ever since I decided to do a magazine I ad on Assassins Creed Brotherhood and an already had an idea in my head and that idea amazing ad on Clash Of Clans. There is is video games. I always loved video games articles on a strategy game called Sid Meiers it gives me something to do it entertains me Civilization 5. My reason for this magazine and it allows me to think and focus on that is to give you fans of this magazine a chance only. Nowadays the best games are the ones to learn more about video games than any online ad can tell you and also its to give you a chance to see the new games coming out or what is starting to be making. Game Enforcer is just a group of people providing you with information and telling you about the latest games. We have great ads that we think you will enjoy and we hope you enjoy them so much you buy them and have fun like so many before. A lot of the games we with the best graphics and action. Everyone likes video games so I thought it would be good to make a magazine on video games. Every person who enjoys video games I expect to buy it and that is my goal get the most sales and the best ratings than any other video game magazine. -
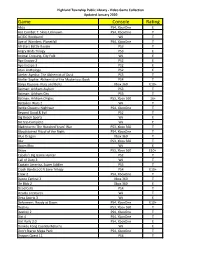
Game Console Rating
Highland Township Public Library - Video Game Collection Updated January 2020 Game Console Rating Abzu PS4, XboxOne E Ace Combat 7: Skies Unknown PS4, XboxOne T AC/DC Rockband Wii T Age of Wonders: Planetfall PS4, XboxOne T All-Stars Battle Royale PS3 T Angry Birds Trilogy PS3 E Animal Crossing, City Folk Wii E Ape Escape 2 PS2 E Ape Escape 3 PS2 E Atari Anthology PS2 E Atelier Ayesha: The Alchemist of Dusk PS3 T Atelier Sophie: Alchemist of the Mysterious Book PS4 T Banjo Kazooie- Nuts and Bolts Xbox 360 E10+ Batman: Arkham Asylum PS3 T Batman: Arkham City PS3 T Batman: Arkham Origins PS3, Xbox 360 16+ Battalion Wars 2 Wii T Battle Chasers: Nightwar PS4, XboxOne T Beyond Good & Evil PS2 T Big Beach Sports Wii E Bit Trip Complete Wii E Bladestorm: The Hundred Years' War PS3, Xbox 360 T Bloodstained Ritual of the Night PS4, XboxOne T Blue Dragon Xbox 360 T Blur PS3, Xbox 360 T Boom Blox Wii E Brave PS3, Xbox 360 E10+ Cabela's Big Game Hunter PS2 T Call of Duty 3 Wii T Captain America, Super Soldier PS3 T Crash Bandicoot N Sane Trilogy PS4 E10+ Crew 2 PS4, XboxOne T Dance Central 3 Xbox 360 T De Blob 2 Xbox 360 E Dead Cells PS4 T Deadly Creatures Wii T Deca Sports 3 Wii E Deformers: Ready at Dawn PS4, XboxOne E10+ Destiny PS3, Xbox 360 T Destiny 2 PS4, XboxOne T Dirt 4 PS4, XboxOne T Dirt Rally 2.0 PS4, XboxOne E Donkey Kong Country Returns Wii E Don't Starve Mega Pack PS4, XboxOne T Dragon Quest 11 PS4 T Highland Township Public Library - Video Game Collection Updated January 2020 Game Console Rating Dragon Quest Builders PS4 E10+ Dragon -

Intersomatic Awareness in Game Design
The London School of Economics and Political Science Intersomatic Awareness in Game Design Siobhán Thomas A thesis submitted to the Department of Management of the London School of Economics for the degree of Doctor of Philosophy. London, June 2015 1 Declaration I certify that the thesis I have presented for examination for the PhD degree of the London School of Economics and Political Science is solely my own work. The copyright of this thesis rests with the author. Quotation from it is permitted, provided that full acknowledgement is made. This thesis may not be reproduced without my prior written consent. I warrant that this authorisation does not, to the best of my belief, infringe the rights of any third party. I declare that my thesis consists of 66,515 words. 2 Abstract The aim of this qualitative research study was to develop an understanding of the lived experiences of game designers from the particular vantage point of intersomatic awareness. Intersomatic awareness is an interbodily awareness based on the premise that the body of another is always understood through the body of the self. While the term intersomatics is related to intersubjectivity, intercoordination, and intercorporeality it has a specific focus on somatic relationships between lived bodies. This research examined game designers’ body-oriented design practices, finding that within design work the body is a ground of experiential knowledge which is largely untapped. To access this knowledge a hermeneutic methodology was employed. The thesis presents a functional model of intersomatic awareness comprised of four dimensions: sensory ordering, sensory intensification, somatic imprinting, and somatic marking. -

ZERO TOLERANCE: Policy on Supporting 1St Party Xbox Games
ZERO TOLERANCE: Policy on Supporting 1st Party Xbox Games FEBRUARY 6, 2009 TRAINING ALERT Target Audience All Xbox Support Agents Introduction This Training Alert is specific to incorrect referral of game issues to game developers and manufacturers. What’s Important Microsoft has received several complaints from our game partners about calls being incorrectly routed to them when the issue should have been resolved by a Microsoft support agent at one of our call centers. This has grown from an annoyance to seriously affecting Microsoft's ability to successfully partner with certain game manufacturers. To respond to this, Microsoft is requesting all call centers to implement a zero tolerance policy on incorrect referrals of Games issues. These must stay within the Microsoft support umbrella through escalations as needed within the call center and on to Microsoft if necessary. Under no circumstances should any T1 support agent, T2 support staff, or supervisor refer a customer with an Xbox game issue back to the game manufacturer, or other external party. If there is any question, escalate the ticket to Microsoft. Please also use the appropriate article First-party game titles include , but are not limited to , the following examples: outlining support • Banjo Kazooie: Nuts & Bolts boundaries and • Fable II definition of 1 st party • Gears of War 2 games. • Halo 3 • Lips VKB Articles: • Scene It? Box Office Smash • Viva Pinata: Trouble In Paradise #910587 First-party game developers include , but are not limited to, the following #917504 examples: • Bungie Studios #910582 • Bizarre Creations • FASA Studios • Rare Studios • Lionhead Studios Again, due to the seriousness of this issue, if any agent fails to adhere to this policy, Microsoft will request that they be permanently removed from the Microsoft account per our policies for other detrimental kinds of actions. -

Score Pause Game Settings Fire Weapon Reload/Action Switch Weapo
Fire Weapon Reload/Action ONLINE ENABLED Switch Weapons Melee Attack Jump Swap Grenades Flashlight Zoom Scope (Click) Throw Grenade E-brake (Warthog) Boost (Vehicles) Crouch (Click) Score Pause Game Settings Get the strategy guide primagames.com® ® 0904 Part No. X10-96235 SAFETY INFORMATION TABLE OF CONTENTS About Photosensitive Seizures Secret Transmission ........................................................................................... 2 A very small percentage of people may experience a seizure when exposed to certain visual images, including flashing lights or patterns that may appear in video games. Even people who Master Chief .......................................................................................................... 3 have no history of seizures or epilepsy may have an undiagnosed condition that can cause these Breakdown of Known Covenant Units ......................................................... 4 “photosensitive epileptic seizures” while watching video games. These seizures may have a variety of symptoms, including lightheadedness, altered vision, eye or Controller ............................................................................................................... 6 face twitching, jerking or shaking of arms or legs, disorientation, confusion, or momentary loss of awareness. Seizures may also cause loss of consciousness or convulsions that can lead to injury from Mjolnir Mark VI Battle Suit HUD ..................................................................... 8 falling down or striking -

Exploring Skill Sets for Computer Game Design Programs Bungie, Inc
Exploring Skill Sets for Computer Game Design Programs Bungie, Inc. Employees Share Their Insights by Markus Geissler, Professor and Deputy Sector Navigator, ICT/Digital Media, Greater Sacramento Region Abstract: Based upon several responses from employees at Bungie, Inc., a developer of computer games based in Seattle, to both general and specific questions about the skills sets required for students who wish to work in the computer game industry, technical knowledge, especially in computer programming, but, more importantly, solid interpersonal skills, the ability to think as part of a team consisting of individuals with varying skill sets, and the strength to solve complex problems should be essential program student learning outcomes (PSLOs) for Computer Game Design or similar academic programs. To that end academic program designers should strongly consider an interdisciplinary preparation which includes coursework that addresses both technical and professional PSLOs and which may be offered by content experts across multiple academic departments. Author’s note: In May 2019 the members of Cosumnes River College’s (CRC) Computer Information Science faculty discussed whether or not to add a Game Design component to the department’s course offerings, possibly to complement existing programs at nearby colleges. To better inform the discussion I volunteered to get some industry input via a family friend who works at Bungie, Inc., an American video game developer based in Bellevue, WA which is the developer of computer games such as Destiny, Halo, Myth, Oni, and Marathon. Several Bungie employees were kind enough to respond first to my initial request for a conversation and then specific questions by CRC’s CIS faculty members which I forwarded to the folks at Bungie. -

Halo Wars 2: Complete Edition” Xbox One X Enhanced Fact Sheet October 2017
“Halo Wars 2: Complete Edition” Xbox One X Enhanced Fact Sheet October 2017 Title: “Halo Wars 2: Complete Edition” Publisher: Microsoft Studios Developer: Creative Assembly / 343 Industries Format: Xbox One Family of Devices and Windows 10 Exclusive ESRB Rating: T for Teen Price2: Complete Edition: $59.99 USD Standard Edition: $39.99 USD Season Pass: $29.99 USD (also included in “Halo Wars 2” Complete Edition) Awakening the Nightmare: $19.99 USD (sold separately from the Season Pass) Availability: November 7, 2017 (Xbox One X enhanced) February 21, 2017 February 17, 2017 for Early Access (included in “Halo Wars 2” Complete Edition) Product Overview: The best-selling console real-time strategy (RTS) of all time is back, complete with Xbox One X enhancements! “Halo Wars 2” is an action-packed RTS on the biggest Halo battlefield ever. Get ready to lead armies of Spartans and other Halo fighting forces like Warthogs, Scorpions, and exciting new units in a brutal war against a terrifying new enemy, The Banished, all in breathtaking 4K resolution and HDR for Xbox One X and Windows 10 PC. “Halo Wars 2: Complete Edition” comes packed with the base game, all Season Pass content and the “Halo Wars 2: Awakening the Nightmare” expansion, making this a great package for anyone who is looking to jump into the “Halo Wars 2” experience. In this long-awaited sequel to the acclaimed “Halo Wars,” a new enemy threatens the Halo universe and the only thing standing between Armageddon and humanity is the brave crew on board the Spirit of Fire. -

Rare Diseases, When Taken Together, Are Are Together, When Taken Diseases, Rare to According at All
2013 MEDICINES IN DEVELOPMENT REPORT Rare Diseases A Report on Orphan Drugs in the Pipeline PRESENTED BY AMERICA’s biopharmACEUTICAL RESEARCH COMPANIES More Than 450 Medicines in Development for Rare Diseases Rare diseases, when taken together, are A major area of this research targets Orphan Drugs in Development* not that rare at all. In fact, according to rare cancers, accounting for more than Application the National Institutes of Health (NIH), one-third of all rare disease medicines in Submitted 30 million Americans have one of the development. Other top research areas Phase III nearly 7,000 diseases that are officially include genetic disorders, neurologi- Phase II deemed “rare” because alone they each cal conditions, infectious diseases and Phase I affect fewer than 200,000 people in autoimmune disorders. the United States. Sometimes, only Despite some recent victories, research a few hundred patients are known to 105 into treatments for rare diseases is a have a particular rare disease. daunting quest. This ongoing innovation Simply receiving a diagnosis of a rare and the hundreds of new medicines in disease often becomes a frustrating development now offer hope that physi- 85 quest, since many doctors may have nev- cians will have new treatment options er before heard of or seen the disease. for patients confronting a rare disease. This is, however, a time of great progress and hope. Biopharmaceutical 65 Contents research is entering an exciting new era Innovative Orphan Drugs with a growing understanding of the in the Pipeline ......................................... 2 human genome. Scientific advances have given researchers new tools to Orphan Drug Approvals ...........................4 explore rare diseases, which are often Challenges in Clinical Trials ......................6 more complex than common diseases. -

Prospectus Amalgamated Rare Earth Mines
41016SE0032 52GMSEa874B1 GENOA 010 PROSPECTUS dated Oth September, I9?i. SECURITIES COMMISSION OR SIMILAR AUTHORITY IN CANADA ILAS IN ANY WAY PASSED UPON THE MERITS OF THE SECURITIES OFFERED HEREUNDER AND ANY REPRESENTATION TO THE CONTRARY IS AN OFFENCE. ;t ,.©t©© Suite 416, 25 Adelaide Street West, Toronto, Ontario. OFFERING: The Company is offering hereunder up to 250,00.0 treasury shares. These shares will be sold by registered security dealers at the market price from time to time, on a best efforts basis with a minimum net return to the Company of 12^cents per share. (SEE HEADING .- THE OPFERING-Page 8 ) The proceeds received by the Company from the ale of treasury shares hereunder will be held in trust by the Company©s registrar and trans fer agent until a minimum of 525,000.00 has been received or for a period of 90 days, whichever occurs first. In the event of fail ure to receive ?25,000.00, all monies received on behalf of the Company shall be returned to the Subscribers. SECONDARY In the event of all the above mentioned treasury .^V.-; OFFERING: shares being sold to the public, Mid-North Engineering Services Limited and Brewis 6 White Limited, (the selling shareholders) will offer, through Registered Securities Dealers, 300,000 shares, the proceeds of which will not accrue to the Company. (SEE HEADING - PRINCIPAL HOLDERS OF SHARES - Page 9.) ^PURPOSE , The purpose of thii. offering is to raise funds ©; OF ISSUE: ,to cover the cost of the Company©s proposed f preliminary exploration programme on its pro perties and for general operating expenses. -
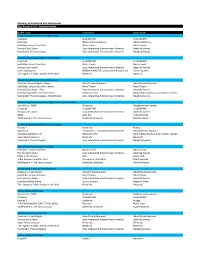
21St Annual DICE Awards Finalists.Xlsx
Academy of Interactive Arts and Sciences 21st Annual D.I.C.E. Awards Finalists GAME TITLE PUBLISHER DEVELOPER Outstanding Achievement in Animation Cuphead StudioMDHR StudioMDHR For Honor Ubisoft Entertainment Ubisoft Montreal Hellblade: Senua's Sacrifice Ninja Theory Ninja Theory Horizon Zero Dawn Sony Interactive Entertainment America Guerrilla Games Uncharted: The Lost Legacy Sony Interactive Entertainment America Naughty Dog LLC Outstanding Achievement in Art Direction Cuphead StudioMDHR StudioMDHR Hellblade: Senua's Sacrifice Ninja Theory Ninja Theory Horizon Zero Dawn Sony Interactive Entertainment America Guerrilla Games Little Nightmares BANDAI NAMCO Entertainment America Inc. Tarsier Studios The Legend of Zelda: Breath of the Wild Nintendo Nintendo Outstanding Achievement in Character Assassin's Creed Origins ‐ Bayek Ubisoft Entertainment Ubisoft Entertainment Hellblade: Senua's Sacrifice ‐ Senua Ninja Theory Ninja Theory Horizon Zero Dawn ‐ Aloy Sony Interactive Entertainment America Guerrilla Games Star Wars Battlefront II ‐ Iden Versio Electronic Arts DICE, Motive Studios, and Criterion Games Uncharted: The Lost Legacy ‐ Chloe Fraiser Sony Interactive Entertainment America Naughty Dog LLC Outstanding Achievement in Original Music Composition Call of Duty: WWII Activision Sledgehammer Games Cuphead StudioMDHR StudioMDHR Horizon Zero Dawn Sony Interactive Entertainment America Guerrilla Games RiME Grey Box Tequila Works Wolfenstein II: The New Colossus Bethesda Softworks MachineGames Outstanding Achievement in Sound Design Destiny -

HALO 3 H2O Brochure
HALO 3 H2O Trace Level Moisture Analyzer GASES & CHEMICALS CEMS ENERGY SEMI & HB LED ATMOSPHERIC LAB & LIFE SCIENCE Designed for trace level moisture analysis, the HALO 3 H2O offers: z Sub parts per billion (ppb) moisture detection capability in an array of gases z Absolute measurement (freedom from calibration gases) z Wide dynamic range—over four orders of magnitude z Low cost of ownership and operational simplicity z Clean technology—no external calibration gases required z Low gas consumption to conserve rare and costly gas z Versatility—trace-level detection in various gas matrices The HALO 3 H2O analyzer provides users with the sensor maintenance, span calibrations, purifier unmatched accuracy, reliability, speed of response replacement and pump rebuilds. As a result, the and ease of operation that users of Tiger Optics HALO 3 H2O analyzer is ideally suited to many analyzers know and expect. Featuring Tiger Optics’ applications where moisture measurement is powerful Cavity Ring-Down Spectroscopy-based extremely critical. These applications include moisture sensor in a very compact and economic fixed bulk gas continuous quality control, portable analyzer design, this versatile analyzer allows users mobile analytical carts, process tool monitoring, air to measure moisture in most inert, corrosive and separation, gas cylinder quality control and many toxic gases with just one device. Users also enjoy other demanding applications. freedom from requirements such as periodic HALO 3 H2O Trace Level Moisture Analyzer Performance Dimensions