Growing Goosenecks: a Study on the Growth and Bioenergetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Goose Barnacle
Fisheries Pêches and Oceans et Océans DFO Science Pacific Region Stock Status Report C6-06 (1998) Rostral - Carinal Length GOOSE BARNACLE Background The Fishery The goose barnacle (Pollicipes polymerus) ranges from southern Alaska to Baja California on the First Nations people have long used goose upper two-thirds of the intertidal zone on exposed or barnacles as food. Goose barnacles have semi-exposed rocky coasts. been commercially harvested sporadically since the 1970s, and continuously since Goose barnacles are hemaphrodidic (one individual has both sexes). They mature at 14-17 mm rostral- 1985. They are hand harvested with various carinal length or one to 3 years of age. Spawning is design cutting tools, and then stored and from late April to early October, with peak spawning shipped as live product. in July, producing 475,000 - 950,000 embryos/adult /season. Larvae are planktonic for 30-40 days, and Goose barnacles have long been recognized settle in suitable habitat at 0.5mm length. as a delicacy in Spain, Portugal and France. Growth is rapid the first year (11-15 mm rostral- The major market for Canadian west coast carinal length) and slows thereafter to 1-3 mm/yr. goose barnacles is Spain, particularly the Maximum size is 45 mm rostral-carinal length, 153 Barcelona area. The market price in Spain peduncle length. Maximum age is unknown. The varies with season and availability from other muscular stalk (peduncle) is analogous to the sources. muscular tail of shrimp, prawns or lobster. Harvesters use a modified cutting and prying tool to Accessibility to the wave swept areas of the free goose barnacles from their substrates and west coast of Vancouver Island (Statistical collect and sort them by hand. -

Snps) in the Northeast Pacific Intertidal Gooseneck Barnacle, Pollicipes Polymerus
University of Alberta New insights about barnacle reproduction: Spermcast mating, aerial copulation and population genetic consequences by Marjan Barazandeh A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Systematics and Evolution Department of Biological Sciences ©Marjan Barazandeh Spring 2014 Edmonton, Alberta Permission is hereby granted to the University of Alberta Libraries to reproduce single copies of this thesis and to lend or sell such copies for private, scholarly or scientific research purposes only. Where the thesis is converted to, or otherwise made available in digital form, the University of Alberta will advise potential users of the thesis of these terms. The author reserves all other publication and other rights in association with the copyright in the thesis and, except as herein before provided, neither the thesis nor any substantial portion thereof may be printed or otherwise reproduced in any material form whatsoever without the author's prior written permission. Abstract Barnacles are mostly hermaphroditic and they are believed to mate via copulation or, in a few species, by self-fertilization. However, isolated individuals of two species that are thought not to self-fertilize, Pollicipes polymerus and Balanus glandula, nonetheless carried fertilized embryo-masses. These observations raise the possibility that individuals may have been fertilized by waterborne sperm, a possibility that has never been seriously considered in barnacles. Using molecular tools (Single Nucleotide Polymorphisms; SNP), I examined spermcast mating in P. polymerus and B. glandula as well as Chthamalus dalli (which is reported to self-fertilize) in Barkley Sound, British Columbia, Canada. -

Five Nations Multi-Species Fishery Management Plan, April 1, 2021
PACIFIC REGION FIVE NATIONS MULTI-SPECIES FISHERY MANAGEMENT PLAN April 1, 2021 – March 31, 2022 SALMON, GROUNDFISH, CRAB, PRAWN, GOOSENECK BARNACLE, AND SEA CUCUMBER Version 1.0 Genus Oncorhynchus Pacific Halibut (Hippoglossus stenolepsis) Gooseneck Barnacle (Pollicipes polymerus) Dungeness crab Sea Cucumber Spot Prawn (Cancer magister) (Apostichopus californicus) (Pandalus platyceros) Fisheries and Oceans Pêches et Océans Canada Canada This Multi-species Fishery Management Plan (FMP) is intended for general purposes only. Where there is a discrepancy between the FMP and the Fisheries Act and Regulations, the Act and Regulations are the final authority. A description of Areas and Subareas referenced in this FMP can be found in the Pacific Fishery Management Area Regulations, 2007. This FMP is not a legally binding instrument which can form the basis of a legal challenge and does not fetter the Minister’s discretionary powers set out in the Fisheries Act. 9-Apr.-21 Version 1.0 Front cover drawing (crab) by Antan Phillips, Retired Biologist, Fisheries and Oceans Canada Front cover drawing (gooseneck barnacle) by Pauline Ridings, Biologist, Fisheries and Oceans Canada Front cover drawing (sea cucumber) by Pauline Ridings, Biologist, Fisheries and Oceans Canada This page intentionally left blank 2021/22 Five Nations Multi-species Fishery Management Plan V. 1.0 Page 2 of 123 9-Apr.-21 Version 1.0 FMP Amendment Tracking Date Version Sections revised and details of revision. 2021-04-09 April 9, 2021 (1.0) Initial 2021/22 Five Nations Multi-species Fishery Management Plan V. 1.0 Page 3 of 123 9-Apr.-21 Version 1.0 CONTENTS Glossary and List of Acronyms .................................................................................................. -
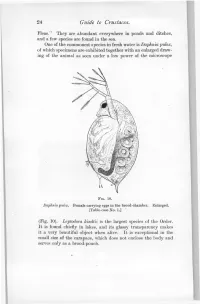
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

Supplementary Materials for Tsunami-Driven Megarafting
Supplementary Materials for Commented [ams1]: Please use SM template provided Tsunami-Driven Megarafting: Transoceanic Species Dispersal and Implications for Marine Biogeography James T. Carlton, John W. Chapman, Jonathan A. Geller, Jessica A. Miller, Deborah A. Carlton, Megan I. McCuller, Nancy C. Treneman, Brian Steves, Gregory M. Ruiz correspondence to: [email protected] This file includes: Materials and Methods Figs. S1 to S8 Tables S1 to S6 1 Material and Methods Sample Acquisition and Processing Following the arrival in June 2012 of a large fishing dock from Misawa and of several Japanese vessels and buoys along the Oregon and Washington coasts (table S1), we established an extensive contact network of local, state, provincial, and federal officials, private citizens, and Commented [ams2]: Can you provide more details, i.e. in what formal sense was this a ‘network’ with nodes and links, rather than a list of contacts? environmental (particularly "coastal cleanup") groups, in Alaska, British Columbia, Washington, Oregon, California, and Hawaii. Between 2012 and 2017 this network grew to hundreds of individuals, many with scientific if not specifically biological training. We advised our contacts that we were interested in acquiring samples of organisms (alive or dead) attached to suspected Japanese Tsunami Marine Debris (JTMD), or to obtain the objects themselves (numerous samples and some objects were received that were North American in origin, or that we interpreted as likely discards from ships-at-sea). We provided detailed directions to searchers and collectors relative to Commented [ams3]: Did you deploy a standard form/protocol for your contacts to use? Can it be included in the SM if so? sample photography, collection, labeling, preservation, and shipping, including real-time communication while investigators were on site. -

Marine Invertebrates of Digha Coast and Some Recomendations on Their Conservation
Rec. zool. Surv. India: 101 (Part 3-4) : 1-23, 2003 MARINE INVERTEBRATES OF DIGHA COAST AND SOME RECOMENDATIONS ON THEIR CONSERVATION RAMAKRISHNA, J A YDIP SARKAR * AND SHANKAR T ALUKDAR Zoological Sruvey of India, M-Block, New Alipore, Kolkata 700 053, India INTRODUCTION The ftrst study on marine fauna of Digha coast is known from the work of Bharati Goswami during 1975-87 (Bharati Goswami, 1992). Other workers, viz., Bairagi, Bhadra, Mukhopadhyaya, Misra, Reddy (1995); Subba Rao et. al., (1992, 1995); Talukdar et. al., (1996); Ramakrishna and Sarkar (1998); Sastry (1995, 1998) and Mitra et. al., (2002) also reported some marine invertebrates under different faunal groups from Hughly-Matla estuary, including Digha. But uptil recently there is no comprehensive updated list of marine invertebrates from Digha coast and adjoining areas. With the establishment of Marine Aquarium and Research Centre, Digha in the year 1990, opportunity was launched for undertaking an extensive exploration and studying seasonal changes that have been taken place on the coastal biodiversity in this area. Accordingly, the authors of the present work, started collecting the detailed faunal infonnation from Digha and adjoining coastal areas [Fig. 2 and 3]. During the study, it has transpired that exploitation of coastal resources has very abruptly increased in recent years. Several new fishing gears are employed, a number of new marine organisms are recognized as commercial fish and non fish resources. Also, the number of trawlers has increased to a large extent. The present paper based on the observations from 1990 to 2000 (including the current records upto January, 2002), is an uptodate database for the available species of marine invertebrates from this area. -

The Role of Avian Predators in an Oregon Rocky Intertidal Community
AN ABSTRACT OF THE THESIS OF Christopher Marsh for the degree of Doctor of Philosophy in Zoology presented on May 24, 1983 Title: The Role of Avian Predators in an Oregon Rocky Intertidal Community Abstract approved: Redacted for Privacy Dr. Bruce-Nenge Birds affected the community structure of an Oregon rocky shore by preying upon mussels (Mytilus spp.) and limpets (Collisella spp.).The impact of such predation is potentially great, as mussels are the competitively dominant mid-intertidal space-occupiers, and limpets are important herbivores in this community. Prey selection by birds reflects differences in bill morphology and foraging tactics. For example, Surfbird (Aphriza virgata) uses its stout bill to tug upright, firmly attached prey (e.g. mussels and gooseneck barnacles [Pollicipes polymerus]) from the substrate. The Black Turnstone (Arenaria melanocephala), with its chisel-shaped bill, uses a hammering tactic to eat firmly attached prey that are 1) compressed in shape and can be dislodged, or 2) have protective shells that can be broken by a turnstone bill. In addition, the Black Turnstone employs a push behavior to feed in clumps of algae containing mobile arthropods. Bird exclusion cages tested the effects of bird predation on 1) rates of mussel recolonization in patches (50 x 50 cm clearings), and 2) densities of small-sized limpets (< 10 mm in length) on upper intertidal mudstone benches. Four of six exclusion experiments showed that birds had a significant effect on mussel recruitment. These experiments suggested that the impact of avian predators had a significant effect on mussel densities when 1) the substrate was relatively smooth,2) other mortality agents were insignificant, and 3) mussels were of intermediate size (11-30 mm long). -

Zootaxa, Pollicipes Caboverdensis Sp. Nov. (Crustacea: Cirripedia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Portal do Conhecimento Zootaxa 2557: 29–38 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) Pollicipes caboverdensis sp. nov. (Crustacea: Cirripedia: Scalpelliformes), an intertidal barnacle from the Cape Verde Islands JOANA N. FERNANDES1, 2, 3, TERESA CRUZ1, 2 & ROBERT VAN SYOC4 1Laboratório de Ciências do Mar, Universidade de Évora, Apartado 190, 7520-903 Sines, Portugal 2Centro de Oceanografia, Faculdade de Ciências da Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal 3Section of Evolution and Ecology, University of California, Davis, CA 95616, USA 4Department of Invertebrate Zoology and Geology, California Academy of Sciences, 55 Music Concourse Dr, San Francisco, CA 94118-4599, USA Abstract Recently, genetic evidence supported the existence of a new species of the genus Pollicipes from the Cape Verde Islands, previously considered a population of P. pollicipes. However, P. pollicipes was not sampled at its southern limit of distribution (Dakar, Senegal), which is geographically separated from the Cape Verde Islands by about 500 km. Herein we describe Pollicipes caboverdensis sp. nov. from the Cape Verde Islands and compare its morphology with the other three species of Pollicipes: P. pollicipes, P. elegans and P. po l y me ru s . Pollicipes pollicipes was sampled at both the middle (Portugal) and southern limit (Dakar, Senegal) of its geographical distribution. The genetic divergence among and within these two regions and Cape Verde was calculated through the analysis of partial mtDNA CO1 gene sequences. -

Fish Bulletin 161. California Marine Fish Landings for 1972 and Designated Common Names of Certain Marine Organisms of California
UC San Diego Fish Bulletin Title Fish Bulletin 161. California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California Permalink https://escholarship.org/uc/item/93g734v0 Authors Pinkas, Leo Gates, Doyle E Frey, Herbert W Publication Date 1974 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY OF CALIFORNIA DEPARTMENT OF FISH AND GAME FISH BULLETIN 161 California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California By Leo Pinkas Marine Resources Region and By Doyle E. Gates and Herbert W. Frey > Marine Resources Region 1974 1 Figure 1. Geographical areas used to summarize California Fisheries statistics. 2 3 1. CALIFORNIA MARINE FISH LANDINGS FOR 1972 LEO PINKAS Marine Resources Region 1.1. INTRODUCTION The protection, propagation, and wise utilization of California's living marine resources (established as common property by statute, Section 1600, Fish and Game Code) is dependent upon the welding of biological, environment- al, economic, and sociological factors. Fundamental to each of these factors, as well as the entire management pro- cess, are harvest records. The California Department of Fish and Game began gathering commercial fisheries land- ing data in 1916. Commercial fish catches were first published in 1929 for the years 1926 and 1927. This report, the 32nd in the landing series, is for the calendar year 1972. It summarizes commercial fishing activities in marine as well as fresh waters and includes the catches of the sportfishing partyboat fleet. Preliminary landing data are published annually in the circular series which also enumerates certain fishery products produced from the catch. -

Kelp Forest Monitoring Handbook — Volume 1: Sampling Protocol
KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL CHANNEL ISLANDS NATIONAL PARK KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL Channel Islands National Park Gary E. Davis David J. Kushner Jennifer M. Mondragon Jeff E. Mondragon Derek Lerma Daniel V. Richards National Park Service Channel Islands National Park 1901 Spinnaker Drive Ventura, California 93001 November 1997 TABLE OF CONTENTS INTRODUCTION .....................................................................................................1 MONITORING DESIGN CONSIDERATIONS ......................................................... Species Selection ...........................................................................................2 Site Selection .................................................................................................3 Sampling Technique Selection .......................................................................3 SAMPLING METHOD PROTOCOL......................................................................... General Information .......................................................................................8 1 m Quadrats ..................................................................................................9 5 m Quadrats ..................................................................................................11 Band Transects ...............................................................................................13 Random Point Contacts ..................................................................................15 -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

Barnacle Paper.PUB
Proc. Isle Wight nat. Hist. archaeol. Soc . 24 : 42-56. BARNACLES (CRUSTACEA: CIRRIPEDIA) OF THE SOLENT & ISLE OF WIGHT Dr Roger J.H. Herbert & Erik Muxagata To coincide with the bicentenary of the birth of the naturalist Charles Darwin (1809-1889) a list of barnacles (Crustacea:Cirripedia) recorded from around the Solent and Isle of Wight coast is pre- sented, including notes on their distribution. Following the Beagle expedition, and prior to the publication of his seminal work Origin of Species in 1859, Darwin spent eight years studying bar- nacles. During this time he tested his developing ideas of natural selection and evolution through precise observation and systematic recording of anatomical variation. To this day, his monographs of living and fossil cirripedia (Darwin 1851a, 1851b, 1854a, 1854b) are still valuable reference works. Darwin visited the Isle of Wight on three occasions (P. Bingham, pers.com) however it is unlikely he carried out any field work on the shore. He does however describe fossil cirripedia from Eocene strata on the Isle of Wight (Darwin 1851b, 1854b) and presented specimens, that were supplied to him by other collectors, to the Natural History Museum (Appendix). Barnacles can be the most numerous of macrobenthic species on hard substrata. The acorn and stalked (pedunculate) barnacles have a familiar sessile adult stage that is preceded by a planktonic larval phase comprising of six naupliar stages, prior to the metamorphosis of a non-feeding cypris that eventually settles on suitable substrate (for reviews on barnacle biology see Rainbow 1984; Anderson, 1994). Additionally, the Rhizocephalans, an ectoparasitic group, are mainly recognis- able as barnacles by the external characteristics of their planktonic nauplii.