Rosettes and Pseudorosettes and Their Significance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central Nervous System Tumors General ~1% of Tumors in Adults, but ~25% of Malignancies in Children (Only 2Nd to Leukemia)
Last updated: 3/4/2021 Prepared by Kurt Schaberg Central Nervous System Tumors General ~1% of tumors in adults, but ~25% of malignancies in children (only 2nd to leukemia). Significant increase in incidence in primary brain tumors in elderly. Metastases to the brain far outnumber primary CNS tumors→ multiple cerebral tumors. One can develop a very good DDX by just location, age, and imaging. Differential Diagnosis by clinical information: Location Pediatric/Young Adult Older Adult Cerebral/ Ganglioglioma, DNET, PXA, Glioblastoma Multiforme (GBM) Supratentorial Ependymoma, AT/RT Infiltrating Astrocytoma (grades II-III), CNS Embryonal Neoplasms Oligodendroglioma, Metastases, Lymphoma, Infection Cerebellar/ PA, Medulloblastoma, Ependymoma, Metastases, Hemangioblastoma, Infratentorial/ Choroid plexus papilloma, AT/RT Choroid plexus papilloma, Subependymoma Fourth ventricle Brainstem PA, DMG Astrocytoma, Glioblastoma, DMG, Metastases Spinal cord Ependymoma, PA, DMG, MPE, Drop Ependymoma, Astrocytoma, DMG, MPE (filum), (intramedullary) metastases Paraganglioma (filum), Spinal cord Meningioma, Schwannoma, Schwannoma, Meningioma, (extramedullary) Metastases, Melanocytoma/melanoma Melanocytoma/melanoma, MPNST Spinal cord Bone tumor, Meningioma, Abscess, Herniated disk, Lymphoma, Abscess, (extradural) Vascular malformation, Metastases, Extra-axial/Dural/ Leukemia/lymphoma, Ewing Sarcoma, Meningioma, SFT, Metastases, Lymphoma, Leptomeningeal Rhabdomyosarcoma, Disseminated medulloblastoma, DLGNT, Sellar/infundibular Pituitary adenoma, Pituitary adenoma, -

Pineal Region Tumors: Computed Tomographic-Pathologic Spectrum
415 Pineal Region Tumors: Computed Tomographic-Pathologic Spectrum Nancy N. Futrell' While several computed tomographic (CT) studies of posterior third ventricular Anne G. Osborn' neoplasms have included descriptions of pineal tumors, few reports have concentrated Bruce D. Cheson 2 on these uncommon lesions. Some authors have asserted that the CT appearance of many pineal tumors is virtually pathognomonic. A series of nine biopsy-proved pineal gland and eight other presumed tumors is presented that illustrates their remarkable heterogeneity in both histopathologic and CT appearance. These tumors included germinomas, teratocarcinomas, hamartomas, and other varieties. They had variable margination, attenuation, calcification, and suprasellar extension. Germinomas have the best response to radiation therapy. Biopsy of pineal region tumors is now feasible and is recommended for treatment planning. Tumors of the pineal region account for less th an 2% of all intracrani al neoplasms [1]. While several reports of computed tomography (CT) of third ventricular neoplasms have in cluded an occasi onal pineal tumor [2 , 3], few have focused on the radiographic spectrum of th ese uncommon lesions [4]. Some authors have asserted that the CT appearance of many pineal tumors is virtuall y pathognomonic [5]. We studied a series of nine biopsy-proven pineal gland tumors that demonstrated remarkable heterogeneity in both histopath ologic and CT appearance. Materials and Methods A total of 17 pineal gland tumors were detected in 15,000 consecutive CT scans. Four patients were female and 13 were male. Mean age for the fe males was 27 years; for the males, 15 years. Initial symptoms ranged from headache, nausea, and vomiting, to Parinaud syndrome, vi sual field defects, diabetes insipidus, and hypopituitari sm (table 1). -

Clinical Radiation Oncology Review
Clinical Radiation Oncology Review Daniel M. Trifiletti University of Virginia Disclaimer: The following is meant to serve as a brief review of information in preparation for board examinations in Radiation Oncology and allow for an open-access, printable, updatable resource for trainees. Recommendations are briefly summarized, vary by institution, and there may be errors. NCCN guidelines are taken from 2014 and may be out-dated. This should be taken into consideration when reading. 1 Table of Contents 1) Pediatrics 6) Gastrointestinal a) Rhabdomyosarcoma a) Esophageal Cancer b) Ewings Sarcoma b) Gastric Cancer c) Wilms Tumor c) Pancreatic Cancer d) Neuroblastoma d) Hepatocellular Carcinoma e) Retinoblastoma e) Colorectal cancer f) Medulloblastoma f) Anal Cancer g) Epndymoma h) Germ cell, Non-Germ cell tumors, Pineal tumors 7) Genitourinary i) Craniopharyngioma a) Prostate Cancer j) Brainstem Glioma i) Low Risk Prostate Cancer & Brachytherapy ii) Intermediate/High Risk Prostate Cancer 2) Central Nervous System iii) Adjuvant/Salvage & Metastatic Prostate Cancer a) Low Grade Glioma b) Bladder Cancer b) High Grade Glioma c) Renal Cell Cancer c) Primary CNS lymphoma d) Urethral Cancer d) Meningioma e) Testicular Cancer e) Pituitary Tumor f) Penile Cancer 3) Head and Neck 8) Gynecologic a) Ocular Melanoma a) Cervical Cancer b) Nasopharyngeal Cancer b) Endometrial Cancer c) Paranasal Sinus Cancer c) Uterine Sarcoma d) Oral Cavity Cancer d) Vulvar Cancer e) Oropharyngeal Cancer e) Vaginal Cancer f) Salivary Gland Cancer f) Ovarian Cancer & Fallopian -
PINEAL REGION TUMORS Onc28 (1)
PINEAL REGION TUMORS Onc28 (1) Pineal Region Tumors, Pineal Parenchymal Tumors Last updated: December 22, 2020 TERMINOLOGY......................................................................................................................................... 1 EPIDEMIOLOGY ........................................................................................................................................ 1 ETIOLOGY ................................................................................................................................................ 1 CLASSIFICATION, PATHOLOGY ............................................................................................................... 1 PINEAL PARENCHYMAL TUMORS ........................................................................................................... 1 Pineocytoma ..................................................................................................................................... 1 Pineoblastoma .................................................................................................................................. 3 Pineal parenchymal tumour of intermediate differentiation ............................................................ 5 Papillary tumour of pineal region ..................................................................................................... 6 GLIOMAS................................................................................................................................................ 7 MISCELLANEOUS -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -

What Can the Neuroradiologist Really Say?
The New World Health Organization Classification of Central Nervous System Tumors: REVIEW ARTICLE What Can the Neuroradiologist Really Say? A.G. Osborn SUMMARY: The WHO Classification of Tumors of the Central Nervous System has become the K.L. Salzman worldwide standard for classifying and grading brain neoplasms. The most recent edition (WHO 2007) introduced a number of significant changes that include both additions and redefinitions or clarifica- M.M. Thurnher tions of existing entities. Eight new neoplasms and 4 new variants were introduced. This article J.H. Rees reviews these entities, summarizing both their histology and imaging appearance. Now with more than M. Castillo 3 years of clinical experience following publication of the newest revision, we also ask, “What can the neuroradiologist really say?” Are there imaging findings that could suggest the preoperative diagnosis of a new tumor entity or variant? ABBREVIATIONS: aCPP ϭ atypical choriod plexus papilloma; CNS ϭ central nervous system; CPP ϭ choriod plexus papilloma; CPCa ϭ choriod plexus carcinoma; DNET ϭ dysembryoplastic neuroep- ithelial tumor; EVNCT ϭ extraventricular neurocytoma; MB ϭ medulloblastoma; MBEN ϭ medul- loblastoma with extensive nodularity; PA ϭ pilocytic astrocytoma; PGNT ϭ papillary glioneuronal tumor; PMA ϭ pilomyxoid astrocytoma; PPTID ϭ pineal parenchymal tumor of intermediate differentiation; PTPRϭ papillary tumor of the pineal region; RGNT ϭ rosette-forming glioneuronal tumor; SCO ϭ spindle cell oncocytoma; T1Cϩϭpost-contrast T1-weighted; T1WI ϭ T1-weighted imaging; T2WI ϭ T2-weighted imaging; WHO ϭ World Health Organization he WHO Classification of Tumors of the Central Nervous mors”) as chordoid glioma and astroblastoma.4 Angiocentric TSystem, now in its fourth edition, is the universal standard gliomas are slowly growing solid hemispheric tumors of chil- 1,2 for classifying and grading brain neoplasms. -

Primary Intracranial Germ Cell Tumor (GCT)
Primary Intracranial Germ Cell Tumor (GCT) Bryce Beard MD, Margaret Soper, MD, and Ricardo Wang, MD Kaiser Permanente Los Angeles Medical Center Los Angeles, California April 19, 2019 Case • 10 year-old boy presents with headache x 2 weeks. • Associated symptoms include nausea, vomiting, and fatigue • PMH/PSH: none • Soc: Lives with mom and dad. 4th grader. Does well in school. • PE: WN/WD. Lethargic. No CN deficits. Normal strength. Dysmetria with finger-to-nose testing on left. April 19, 2019 Presentation of Intracranial GCTs • Symptoms depend on location of tumor. – Pineal location • Acute onset of symptoms • Symptoms of increased ICP due to obstructive hydrocephalus (nausea, vomiting, headache, lethargy) • Parinaud’s syndrome: Upward gaze and convergence palsy – Suprasellar location: • Indolent onset of symptoms • Endocrinopathies • Visual field deficits (i.e. bitemporal hemianopsia) – Diabetes insipidus can present due to tumor involvement of either location. – 2:1 pineal:suprasellar involvement. 5-10% will present with both (“bifocal germinoma”). April 19, 2019 Suprasellar cistern Anatomy 3rd ventricle Pineal gland Optic chiasm Quadrigeminal Cistern Cerebral (Sylvian) aquaduct Interpeduncular Cistern 4th ventricle Prepontine Cistern April 19, 2019 Anatomy Frontal horn of rd lateral ventricle 3 ventricle Interpeduncular cistern Suprasellar cistern Occipital horn of lateral Quadrigeminal Ambient ventricle cistern cistern April 19, 2019 Case CT head: Hydrocephalus with enlargement of lateral and 3rd ventricles. 4.4 x 3.3 x 3.3 cm midline mass isodense to grey matter with calcifications. April 19, 2019 Case MRI brain: Intermediate- to hyper- intense 3rd ventricle/aqueduct mass with heterogenous enhancement. April 19, 2019 Imaging Characteristics • Imaging cannot reliably distinguish different types of GCTs, however non-germinomatous germ cell tumors (NGGCTs) tend to have more heterogenous imaging characteristics compared to germinomas. -

WHO Classification of Tumors of the Central Nervous System
Appendix A: WHO Classification of Tumors of the Central Nervous System WHO Classification 1979 • Ganglioneuroblastoma • Anaplastic [malignant] gangliocytoma and Zülch KJ (1979) Histological typing of tumours ganglioglioma of the central nervous system. 1st ed. World • Neuroblastoma Health Organization, Geneva • Poorly differentiated and embryonal tumours • Glioblastoma Tumours of Neuroepithelial tissue –– Variants: • Astrocytic tumours –– Glioblastoma with sarcomatous compo- • Astrocytoma nent [mixed glioblastoma and sarcoma] –– fibrillary –– Giant cell glioblastoma –– protoplasmic • Medulloblastoma –– gemistocytic –– Variants: • Pilocytic astrocytoma –– desmoplastic medulloblastoma • Subependymal giant cell astrocytoma [ven- –– medullomyoblastoma tricular tumour of tuberous sclerosis] • Medulloepithelioma • Astroblastoma • Primitive polar spongioblastoma • Anaplastic [malignant] astrocytoma • Gliomatosis cerebri • Oligodendroglial tumours • Oligodendroglioma Tumours of nerve sheath cells • Mixed-oligo-astrocytoma • Neurilemmoma [Schwannoma, neurinoma] • Anaplastic [malignant] oligodendroglioma • Anaplastic [malignant] neurilemmoma [schwan- • Ependymal and choroid plexus tumours noma, neurinoma] • Ependymoma • Neurofibroma –– Myxopapillary • Anaplastic [malignant]neurofibroma [neurofi- –– Papillary brosarcoma, neurogenic sarcoma] –– Subependymoma • Anaplastic [malignant] ependymoma Tumours of meningeal and related tissues • Choroid plexus papilloma • Meningioma • Anaplastic [malignant] choroid plexus papil- –– meningotheliomatous [endotheliomatous, -

2018 Solid Tumor Rules Lois Dickie, CTR, Carol Johnson, BS, CTR (Retired), Suzanne Adams, BS, CTR, Serban Negoita, MD, Phd
Solid Tumor Rules Effective with Cases Diagnosed 1/1/2018 and Forward Updated November 2020 Editors: Lois Dickie, CTR, NCI SEER Carol Hahn Johnson, BS, CTR (Retired), Consultant Suzanne Adams, BS, CTR (IMS, Inc.) Serban Negoita, MD, PhD, CTR, NCI SEER Suggested citation: Dickie, L., Johnson, CH., Adams, S., Negoita, S. (November 2020). Solid Tumor Rules. National Cancer Institute, Rockville, MD 20850. Solid Tumor Rules 2018 Preface (Excludes lymphoma and leukemia M9590 – M9992) In Appreciation NCI SEER gratefully acknowledges the dedicated work of Dr. Charles Platz who has been with the project since the inception of the 2007 Multiple Primary and Histology Coding Rules. We appreciate the support he continues to provide for the Solid Tumor Rules. The quality of the Solid Tumor Rules directly relates to his commitment. NCI SEER would also like to acknowledge the Solid Tumor Work Group who provided input on the manual. Their contributions are greatly appreciated. Peggy Adamo, NCI SEER Elizabeth Ramirez, New Mexico/SEER Theresa Anderson, Canada Monika Rivera, New York Mari Carlos, USC/SEER Jennifer Ruhl, NCI SEER Louanne Currence, Missouri Nancy Santos, Connecticut/SEER Frances Ross, Kentucky/SEER Kacey Wigren, Utah/SEER Raymundo Elido, Hawaii/SEER Carolyn Callaghan, Seattle/SEER Jim Hofferkamp, NAACCR Shawky Matta, California/SEER Meichin Hsieh, Louisiana/SEER Mignon Dryden, California/SEER Carol Kruchko, CBTRUS Linda O’Brien, Alaska/SEER Bobbi Matt, Iowa/SEER Mary Brandt, California/SEER Pamela Moats, West Virginia Sarah Manson, CDC Patrick Nicolin, Detroit/SEER Lynda Douglas, CDC Cathy Phillips, Connecticut/SEER Angela Martin, NAACCR Solid Tumor Rules 2 Updated November 2020 Solid Tumor Rules 2018 Preface (Excludes lymphoma and leukemia M9590 – M9992) The 2018 Solid Tumor Rules Lois Dickie, CTR, Carol Johnson, BS, CTR (Retired), Suzanne Adams, BS, CTR, Serban Negoita, MD, PhD Preface The 2007 Multiple Primary and Histology (MPH) Coding Rules have been revised and are now referred to as 2018 Solid Tumor Rules. -

Chapter 4: Histological Groups
Chapter 4: Histological groups Jacques Ferlay and Brian Rous BACKGROUND at these sites are adenocarcinomas, and the coding of The data presented in this volume of Cancer Incidence adenocarcinoma subtypes is not consistent. Kidney in Five Continents (CI5) are mainly organized by the was combined with renal pelvis because some predominantly site-based categories of Chapter II urothelial carcinomas originating from the renal pelvis (Neoplasms) of the 10th revision of the International are site coded as kidney cancers, while others are Classification of Diseases (ICD-10; WHO, 1992) (see coded as renal pelvis cancers. Hodgkin lymphoma Chapter 3 of this book). However, for some sites of and leukaemias are combined together in the cancer, the histological type is particularly relevant haematological group. clinically and/or epidemiologically. In the present volume, the data are grouped according to histological TYPES OF NEOPLASMS INCLUDED types as originally defined in CI5 Volume IX (Curado et The CI5 Volume X data include all invasive malignant al., 2007) and listed in this chapter. neoplasms and some non-invasive malignant neoplasms. For most morphology codes, a fifth digit of GENERAL STRUCTURE /1 or /2 automatically excludes data entry. Carcinoma The main structure of the histological grouping is that in situ is reported by many cancer registries, but is not specific types of malignant neoplasms are listed, as generally dealt with in this volume, with the exception well as the category “Unspecified malignant neoplasm” of urothelial carcinoma in situ. A few lesions of (i.e. malignant tumours that are so poorly differentiated borderline malignancy are included, such as low-grade that we are unable to classify them into major groups non-invasive papillary transitional cell tumours, which such as carcinoma or sarcoma). -

NYS Cancer Registry Facility Reporting Manual
The New York State CANCER REGISTRY Facility Reporting Manual 2021 - EDITION THE NEW YORK STATE DEPARTMENT OF HEALTH STATE OF NEW YORK KATHY HOCHUL, GOVERNOR DEPARTMENT OF HEALTH HOWARD A. ZUCKER, M.D., J.D., COMMISSIONER The NYSCR Reporting Manual Revised September 2021 New York State Cancer Registry Reporting Manual Table of Contents ACKNOWLEDGEMENT PART ONE – OVERVIEW PART TWO – CONFIDENTIALITY PART THREE - REPORTABLE CONDITIONS AND TERMINOLOGY PART FOUR - DATA ITEMS AND DESCRIPTIONS PART FIVE - CASEFINDING PART SIX - DEATH CERTIFICATE ONLY AND DEATH CLEARANCE LISTS PART SEVEN – QUALITY ASSURANCE PART EIGHT – ELECTRONIC REPORTING APPENDIX A - NYS PUBLIC HEALTH LAW APPENDIX B – HIPAA INFORMATION The NYSCR Reporting Manual – Table of Contents Revised September 2021 Page Left Blank Intentionally The NYSCR Reporting Manual Revised September 2021 ACKNOWLEDGEMENT We wish to acknowledge the Centers for Disease Control and Prevention's (CDC) National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Surveillance Epidemiology and End Results program (SEER) for their support. Production of this Reporting Manual was supported in part by a cooperative agreement awarded to the New York State Department of Health by the NPCR and a contract with SEER. Its contents are solely the responsibility of the New York State Department of Health and do not necessarily represent the official views of the CDC or NCI. The NYSCR Reporting Manual - Acknowledgement Revised September 2021 Page Left Blank Intentionally The NYSCR Reporting Manual Revised September 2021 New York State Cancer Registry Reporting Manual Part One – Overview 1.1 WHAT IS THE NEW YORK STATE CANCER REGISTRY? .................................... 1 1.2 WHY REPORT TO THE NYSCR? .......................................................................... -
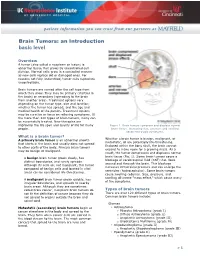
Brain Tumors: an Introduction Basic Level
Brain Tumors: an Introduction basic level Overview A tumor (also called a neoplasm or lesion) is abnormal tissue that grows by uncontrolled cell division. Normal cells grow in a controlled manner as new cells replace old or damaged ones. For reasons not fully understood, tumor cells reproduce uncontrollably. Brain tumors are named after the cell type from which they grow. They may be primary (starting in the brain) or secondary (spreading to the brain from another area). Treatment options vary depending on the tumor type, size and location; whether the tumor has spread; and the age and medical health of the person. Treatment options may be curative or focus on relieving symptoms. Of the more than 120 types of brain tumors, many can be successfully treated. New therapies are improving the life span and quality of life for many Figure 1. Brain tumors compress and displace normal brain tissue. Increasing size, pressure and swelling people. cause neurologic symptoms. What is a brain tumor? Whether a brain tumor is benign, malignant, or A primary brain tumor is an abnormal growth metastatic, all are potentially life-threatening. that starts in the brain and usually does not spread Enclosed within the bony skull, the brain cannot to other parts of the body. Primary brain tumors expand to make room for a growing mass. As a may be benign or malignant. result, the tumor compresses and displaces normal brain tissue (Fig. 1). Some brain tumors cause a A benign brain tumor grows slowly, has blockage of cerebrospinal fluid (CSF) that flows distinct boundaries, and rarely spreads.