Structural and Functional Characteristics of 53BP1-Dependent DNA Repair
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Localization of Condensin Subunit XCAP-E in Interphase Nucleus, Nucleoid and Nuclear
1 Localization of condensin subunit XCAP-E in interphase nucleus, nucleoid and nuclear matrix of XL2 cells. Elmira Timirbulatova, Igor Kireev, Vladimir Ju. Polyakov, and Rustem Uzbekov* Division of Electron Microscopy, A.N.Belozersky Institute of Physico-Chemical Biology, Moscow State University, 119899, Moscow, Russia. *Author for correspondence: telephone. 007-095-939-55-28; FAX 007-095-939-31-81 e-mail: [email protected] Key words: XCAP-E; nucleolus; condensin; nuclear matrix; Xenopus. Abbreviations: DAPI , 4’, 6 diamidino-2-phenylindole; DNP, deoxyribonucleoprotein; DRB, 5,6-dichloro-1b-d-ribofuranosylbenzimidazole; SMC, structural maintenance of chromosomes; XCAP-E, Xenopus chromosome associated protein E. 2 Abstract The Xenopus XCAP-E protein is a component of condensin complex In the present work we investigate its localization in interphase XL2 cells and nucleoids. We shown, that XCAP-E is localizes in granular and in dense fibrillar component of nucleolus and also in small karyoplasmic structures (termed “SMC bodies”). Extraction by 2M NaCl does not influence XCAP-E distribution in nucleolus and “SMC bodies”. DNAse I treatment of interphase cells permeabilized by Triton X-100 or nucleoids resulted in partial decrease of labeling intensity in the nucleus, whereas RNAse A treatment resulted in practically complete loss of labeling of nucleolus and “SMC bodies” labeling. In mitotic cells, however, 2M NaCl extraction results in an intense staining of the chromosome region although the labeling was visible along the whole length of sister chromatids, with a stronger staining in centromore region. The data are discussed in view of a hypothesis about participation of XCAP-E in processing of ribosomal RNA. -

Monoclonal Antibody to SMC2 / CAPE - Purified
OriGene Technologies, Inc. OriGene Technologies GmbH 9620 Medical Center Drive, Ste 200 Schillerstr. 5 Rockville, MD 20850 32052 Herford UNITED STATES GERMANY Phone: +1-888-267-4436 Phone: +49-5221-34606-0 Fax: +1-301-340-8606 Fax: +49-5221-34606-11 [email protected] [email protected] AM05324PU-N Monoclonal Antibody to SMC2 / CAPE - Purified Alternate names: CAP-E, Chromosome-associated protein E, SMC protein 2, SMC-2, SMC2L1, Structural maintenance of chromosomes protein 2, XCAP-E homolog Quantity: 0.1 mg Concentration: 1.0 mg/ml Background: CAP-E and CAP-C (Chromosome Associated Protein-E & C) are also SMC (Structural Maintenance of Chromosome) family members that form a heterodimeric complex required for mitotic chromosome condensation to achieve proper segregation of genetic information during subsequent cell division. hCAP-C/hCAP-E is a component of a multiprotein complex called condensin that interacts with phosphorylation of Histone H3 resulting in the mitotic chromosome condensation. The distribution patterns of the two heterodimeric complexes in interphase nucleus indicate independent behaviour of the two complexes during cell cycle. These results suggest that the two distinct complexes are involved in different aspects of mitotic chromosome organization in human cells. Uniprot ID: O95347 NCBI: NP_001036015.1 GeneID: 10592 Host / Isotype: Mouse / IgG Clone: E1M Immunogen: Hybridoma produced by the fusion of splenocytes from mice immunized with recombinant protein corresponding to amino acids 523-768 of Human CAP-E and Mouse myeloma cells. Genename: SMC2 Format: State: Liquid purified IgG fraction. Buffer System: PBS containing 0.08% Sodium Azide as preservative. Applications: Western Blot: 1-5 µg/ml. -

Supplementary Table S1. Correlation Between the Mutant P53-Interacting Partners and PTTG3P, PTTG1 and PTTG2, Based on Data from Starbase V3.0 Database
Supplementary Table S1. Correlation between the mutant p53-interacting partners and PTTG3P, PTTG1 and PTTG2, based on data from StarBase v3.0 database. PTTG3P PTTG1 PTTG2 Gene ID Coefficient-R p-value Coefficient-R p-value Coefficient-R p-value NF-YA ENSG00000001167 −0.077 8.59e-2 −0.210 2.09e-6 −0.122 6.23e-3 NF-YB ENSG00000120837 0.176 7.12e-5 0.227 2.82e-7 0.094 3.59e-2 NF-YC ENSG00000066136 0.124 5.45e-3 0.124 5.40e-3 0.051 2.51e-1 Sp1 ENSG00000185591 −0.014 7.50e-1 −0.201 5.82e-6 −0.072 1.07e-1 Ets-1 ENSG00000134954 −0.096 3.14e-2 −0.257 4.83e-9 0.034 4.46e-1 VDR ENSG00000111424 −0.091 4.10e-2 −0.216 1.03e-6 0.014 7.48e-1 SREBP-2 ENSG00000198911 −0.064 1.53e-1 −0.147 9.27e-4 −0.073 1.01e-1 TopBP1 ENSG00000163781 0.067 1.36e-1 0.051 2.57e-1 −0.020 6.57e-1 Pin1 ENSG00000127445 0.250 1.40e-8 0.571 9.56e-45 0.187 2.52e-5 MRE11 ENSG00000020922 0.063 1.56e-1 −0.007 8.81e-1 −0.024 5.93e-1 PML ENSG00000140464 0.072 1.05e-1 0.217 9.36e-7 0.166 1.85e-4 p63 ENSG00000073282 −0.120 7.04e-3 −0.283 1.08e-10 −0.198 7.71e-6 p73 ENSG00000078900 0.104 2.03e-2 0.258 4.67e-9 0.097 3.02e-2 Supplementary Table S2. -

Lorena Novoa-Aponte
A Strain: fl/fl ∆hep Strain: fl/fl ∆hep AnalysisAAV: ofLuc the ironLuc chaperonePCBP1-WT PCBP1PCBP1-∆Fe interactome:PCBP1-∆RNA PCBP1 variant intersection of DNA repair and iron traffickingAAV: Luc Luc WT ∆Fe ∆RNA PCBP1 −40 Lorena Novoa-Aponte a*, Sarju J. Patel a, Olga Protchenko a, James Wohlschlegel b, Caroline C. Philpott a −40 PCBP1, IHC PCBP1, GAPDH - a Genetics and Metabolism Section,a NIDDK, NIH, Bethesda, MD, USA. b Department of Biological Chemistry, UCLA, Los Angeles, CA, USA C.C. Philpott, et al. *[email protected] 80 B P = 0.0262 60 40 1. Iron is essential but toxic 2. Increased DNA damage in cells lacking PCBP1 ns H&E ? + 20 C.C. Philpott, et al. Iron is used as an essential cofactor by several enzymes involved in DNA ⦿ Increased TUNEL in cells and tissue livers from mice lacking PCBP1. ? replication and repair. However, unchaperoned iron promotes redox ⦿ PCBP1 binds both, iron and single-stranded nucleicTG, nmol/mg protein 0 acids. stress that may affect DNA stability. Strain: Δhep Δhep Δhep ? ⦿ The iron binding activity of PCBP1 controls suppressionAAV: of DNA damage C WT ∆Fe ∆RNA PCBP1 variant ? Iron storage HEK293 cells Mouse Liver ? A 8 siNT+P1var 100 ? A 8 var siPCBP1 2 ? Mammals use the iron siNT+P1 ns var Fe-S cluster assembly 6 siPCBP1+P1siPCBP1 80 ADI1 TUNEL chaperone PCBP1 to var ? 6 siPCBP1+P1 metalate several iron- 60 = 0.0468 = 0.0354 = 0.0465 cell / mm population P P P population + dependent enzymes + 4 + 4 ? 40 Degradation of HIF1α Fig. 3. Iron chaperone-mediated handling of cytosolic labile iron pool. -

A Yeast Phenomic Model for the Influence of Warburg Metabolism on Genetic Buffering of Doxorubicin Sean M
Santos and Hartman Cancer & Metabolism (2019) 7:9 https://doi.org/10.1186/s40170-019-0201-3 RESEARCH Open Access A yeast phenomic model for the influence of Warburg metabolism on genetic buffering of doxorubicin Sean M. Santos and John L. Hartman IV* Abstract Background: The influence of the Warburg phenomenon on chemotherapy response is unknown. Saccharomyces cerevisiae mimics the Warburg effect, repressing respiration in the presence of adequate glucose. Yeast phenomic experiments were conducted to assess potential influences of Warburg metabolism on gene-drug interaction underlying the cellular response to doxorubicin. Homologous genes from yeast phenomic and cancer pharmacogenomics data were analyzed to infer evolutionary conservation of gene-drug interaction and predict therapeutic relevance. Methods: Cell proliferation phenotypes (CPPs) of the yeast gene knockout/knockdown library were measured by quantitative high-throughput cell array phenotyping (Q-HTCP), treating with escalating doxorubicin concentrations under conditions of respiratory or glycolytic metabolism. Doxorubicin-gene interaction was quantified by departure of CPPs observed for the doxorubicin-treated mutant strain from that expected based on an interaction model. Recursive expectation-maximization clustering (REMc) and Gene Ontology (GO)-based analyses of interactions identified functional biological modules that differentially buffer or promote doxorubicin cytotoxicity with respect to Warburg metabolism. Yeast phenomic and cancer pharmacogenomics data were integrated to predict differential gene expression causally influencing doxorubicin anti-tumor efficacy. Results: Yeast compromised for genes functioning in chromatin organization, and several other cellular processes are more resistant to doxorubicin under glycolytic conditions. Thus, the Warburg transition appears to alleviate requirements for cellular functions that buffer doxorubicin cytotoxicity in a respiratory context. -

The Genetic Program of Pancreatic Beta-Cell Replication in Vivo
Page 1 of 65 Diabetes The genetic program of pancreatic beta-cell replication in vivo Agnes Klochendler1, Inbal Caspi2, Noa Corem1, Maya Moran3, Oriel Friedlich1, Sharona Elgavish4, Yuval Nevo4, Aharon Helman1, Benjamin Glaser5, Amir Eden3, Shalev Itzkovitz2, Yuval Dor1,* 1Department of Developmental Biology and Cancer Research, The Institute for Medical Research Israel-Canada, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel 2Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel. 3Department of Cell and Developmental Biology, The Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Jerusalem 91904, Israel 4Info-CORE, Bioinformatics Unit of the I-CORE Computation Center, The Hebrew University and Hadassah, The Institute for Medical Research Israel- Canada, The Hebrew University-Hadassah Medical School, Jerusalem 91120, Israel 5Endocrinology and Metabolism Service, Department of Internal Medicine, Hadassah-Hebrew University Medical Center, Jerusalem 91120, Israel *Correspondence: [email protected] Running title: The genetic program of pancreatic β-cell replication 1 Diabetes Publish Ahead of Print, published online March 18, 2016 Diabetes Page 2 of 65 Abstract The molecular program underlying infrequent replication of pancreatic beta- cells remains largely inaccessible. Using transgenic mice expressing GFP in cycling cells we sorted live, replicating beta-cells and determined their transcriptome. Replicating beta-cells upregulate hundreds of proliferation- related genes, along with many novel putative cell cycle components. Strikingly, genes involved in beta-cell functions, namely glucose sensing and insulin secretion were repressed. Further studies using single molecule RNA in situ hybridization revealed that in fact, replicating beta-cells double the amount of RNA for most genes, but this upregulation excludes genes involved in beta-cell function. -

SMC1 and SMC3 Proteins in Meiosis 675 at 37¡C, Followed by 12-48 Hours at 4¡C
Journal of Cell Science 113, 673-682 (2000) 673 Printed in Great Britain © The Company of Biologists Limited 2000 JCS0916 Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes M. Eijpe1, C. Heyting1, B. Gross2 and R. Jessberger2,* 1Department of Biomolecular Sciences, Laboratory of Genetics, Wageningen University, NL-6703 HA, Wageningen, The Netherlands 2Basel Institute for Immunology, Grenzacherstr. 487, CH-4058 Basel, Switzerland *Author for correspondence (e-mail: [email protected]) Accepted 1 December 1999; published on WWW 31 January 2000 SUMMARY In somatic cells, the heterodimeric Structural Maintenance complexes of pachytene and diplotene chromosomes. Both of Chromosomes (SMC) proteins are involved in SMC proteins are present in rat spermatocytes and chromosome condensation and gene dosage compensation enriched in preparations of synaptonemal complexes. (SMC2 and 4), and sister chromatid cohesion and DNA Several independent experimental approaches revealed recombination (SMC1 and 3). We report here evidence for interactions of the SMC proteins with synaptonemal an involvement of mammalian SMC1 and SMC3 proteins complex-specific proteins SCP2 and SCP3. These results in meiosis. Immunofluorescence analysis of testis sections suggest a model for the arrangement of SMC proteins in showed intense chromatin association in meiotic prophase mammalian meiotic chromatin. cells, weaker staining in round spermatids and absence of the SMC proteins in elongated spermatids. In spermatocyte nuclei spreads, the SMC1 and SMC3 proteins localize in a Key words: SMC protein, Meiosis, Synaptonemal complex, SCP beaded structure along the axial elements of synaptonemal protein, Testis INTRODUCTION protein complexes with different functions. Genetic studies in S. cerevisiae revealed a requirement for the SMC1/3 proteins The evolutionarily well conserved eukaryotic Structural in mitotic sister chromatid cohesion (Guacci et al., 1997; Maintenance of Chromosomes (SMC) protein family, with four Michaelis et al., 1997). -

Condensin: Crafting the Chromosome Landscape Ilaria Piazza, Christian H
Condensin: crafting the chromosome landscape Ilaria Piazza, Christian H. Haering, Anna Rutkowska Cell Biology & Biophysics Unit, Structural & Computational Biology Unit, European Molecular Biology Laboratory (EMBL) Meyerhofstr. 1, 69117 Heidelberg, Germany C. H. Haering, [email protected], A. Rutkowska, [email protected] This is the unedited version of the manuscript published in final form in Chromosoma Volume 122, Issue 3, 175-190, 2 April 2013 The successful transmission of complete genomes from mother to daughter cells during cell divisions requires the structural re-organization of chromosomes into individualized and compact structures that can be segregated by mitotic spindle microtubules. The multi-subunit condensin protein complex plays a central part in this chromosome condensation process, but the mechanisms behind its actions are still poorly understood. An increasing body of evidence suggests that, in addition to their role in shaping mitotic chromosomes, condensin complexes have important functions in directing the three-dimensional arrangement of chromatin fibers within the interphase nucleus. To fulfill their different functions in genome organization, the activity of condensin complexes and their localization on chromosomes need to be strictly controlled. In this review article, we outline the regulation of condensin function by phosphorylation and other posttranslational modifications at different stages of the cell cycle. We furthermore discuss how these regulatory mechanisms are used to control condensin -

C6cc08797c1.Pdf
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is © The Royal Society of Chemistry 2017 Supplementary Information for Competition-based, quantitative chemical proteomics in breast cancer cells identifies new target profiles for sulforaphane James A. Clulow,a,‡ Elisabeth M. Storck,a,‡ Thomas Lanyon-Hogg,a,‡ Karunakaran A. Kalesh,a Lyn Jonesb and Edward W. Tatea,* a Department of Chemistry, Imperial College London, London, UK, SW7 2AZ b Worldwide Medicinal Chemistry, Pfizer, 200 Cambridge Park Drive, MA 02140, USA ‡ Authors share equal contribution * corresponding author, email: [email protected] Table of Contents 1 Supporting Figures..........................................................................................................................3 2 Supporting Tables.........................................................................................................................17 2.1 Supporting Table S1. Incorporation validation for the R10K8 label in the 'spike-in' SILAC proteome of the MCF7 cell line........................................................................................................17 2.2 Supporting Table S2. Incorporation validation for the R10K8 label in the 'spike-in' SILAC proteome of the MDA-MB-231 cell line ...........................................................................................17 2.3 Supporting Table S3. High- and medium- confidence targets of sulforaphane in the MCF7 cell line..............................................................................................................................................17 -
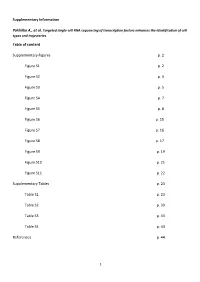
Supplementary Information Table of Content Supplementary Figures P. 2 Figure S1 P. 2 Figure S2 P. 3 Figure S3 P. 5 Figure S4 P
Supplementary Information Pokhilko A., et al. Targeted single-cell RNA sequencing of transcription factors enhances the identification of cell types and trajectories Table of content Supplementary Figures p. 2 Figure S1 p. 2 Figure S2 p. 3 Figure S3 p. 5 Figure S4 p. 7 Figure S5 p. 8 Figure S6 p. 15 Figure S7 p. 16 Figure S8 p. 17 Figure S9 p. 19 Figure S10 p. 21 Figure S11 p. 22 Supplementary Tables p. 23 Table S1 p. 23 Table S2 p. 39 Table S3 p. 43 Table S4 p. 44 References p. 44 1 Figure S1. Capture-Seq improves the quality of scRNA-seq libraries and enriches for targeted TFs. a, Post-capture enrichment of 585 captured TFs detected pre-capture. For each gene the enrichment was calculated a ratio between the average CPMs in the post- and pre-capture libraries, plotted in log2 scale after adding a pseudocount of 1(Curion et al. 2020). b, Linear correlation between the pre- and post-capture averaged expression (raw counts) of 585 common TFs. ERCC spike-ins targeted by scCapture-seq are shown in red. a b 2 Figure S2. Characterization of cell clusters in pre- and post-capture libraries. a,b, Heat maps of cell type TF markers, which were differentially expressed post-capture (b), and their pre-capture expression (a). c,d, Heat maps of TF DEGs, which were differentially expressed post-capture (d,e) and their expression in pre-capture (c). Cells are ordered by post-capture clusters on a-d, as annotated above the heatmaps. -

NCAPG Dynamically Coordinates the Myogenesis of Fetal Bovine Tissue by Adjusting Chromatin Accessibility
International Journal of Molecular Sciences Article NCAPG Dynamically Coordinates the Myogenesis of Fetal Bovine Tissue by Adjusting Chromatin Accessibility 1,2, 1, 3 4 1 1 1 Xin Hu y, Yishen Xing y, Xing Fu , Qiyuan Yang , Ling Ren , Yahui Wang , Qian Li , Junya Li 1,* and Lupei Zhang 1,* 1 Key Laboratory of Animal Genetics Breeding and Reproduction, Ministry of Agriculture and Rural Affairs; Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China; [email protected] (X.H.); [email protected] (Y.X.); [email protected] (L.R.); [email protected] (Y.W.); [email protected] (Q.L.) 2 Molecular and Cellular Biology, Gembloux Agro-Bio Tech, University of Liège, 5030 Gembloux, Belgium 3 School of Animal Sciences, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA; [email protected] 4 Department of Molecular, Cell and Cancer Biology, University of Massachusetts Medical School, Worcester, MA 01655, USA; [email protected] * Correspondence: [email protected] (L.Z.); [email protected] (J.L.) These authors contributed equally to this work. y Received: 29 January 2020; Accepted: 11 February 2020; Published: 13 February 2020 Abstract: NCAPG is a subunit of condensin I that plays a crucial role in chromatin condensation during mitosis. NCAPG has been demonstrated to be associated with farm animal growth traits. However, its role in regulating myoblast differentiation is still unclear. We used myoblasts derived from fetal bovine tissue as an in vitro model and found that NCAPG was expressed during myogenic differentiation in the cytoplasm and nucleus. Silencing NCAPG prolonged the mitosis and impaired the differentiation due to increased myoblast apoptosis. -

Protein List
Protein Accession Protein Id Protein Name P11171 41 Protein 4.