Module 8 : Carbonyls and Phosphine Complexes Lecture 1 : Metal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Ligand Complexation Reactions On
Organic ligand complexation reactions on aluminium-bearing mineral surfaces studied via in-situ Multiple Internal Reflection Infrared Spectroscopy, adsorption experiments, and surface complexation modelling A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences 2010 Charalambos Assos School of Earth, Atmospheric and Environmental Sciences Table of Contents LIST OF FIGURES ......................................................................................................4 LIST OF TABLES ........................................................................................................8 ABSTRACT.................................................................................................................10 DECLARATION.........................................................................................................11 COPYRIGHT STATEMENT....................................................................................12 CHAPTER 1 INTRODUCTION ...............................................................................13 AIMS AND OBJECTIVES .................................................................................................38 CHAPTER 2 THE USE OF IR SPECTROSCOPY IN THE STUDY OF ORGANIC LIGAND SURFACE COMPLEXATION............................................40 INTRODUCTION.............................................................................................................40 METHODOLOGY ...........................................................................................................44 -

Crystal Field Theory (CFT)
Crystal Field Theory (CFT) The bonding of transition metal complexes can be explained by two approaches: crystal field theory and molecular orbital theory. Molecular orbital theory takes a covalent approach, and considers the overlap of d-orbitals with orbitals on the ligands to form molecular orbitals; this is not covered on this site. Crystal field theory takes the ionic approach and considers the ligands as point charges around a central metal positive ion, ignoring any covalent interactions. The negative charge on the ligands is repelled by electrons in the d-orbitals of the metal. The orientation of the d orbitals with respect to the ligands around the central metal ion is important, and can be used to explain why the five d-orbitals are not degenerate (= at the same energy). Whether the d orbitals point along or in between the cartesian axes determines how the orbitals are split into groups of different energies. Why is it required? The valence bond approach could not explain the Electronic spectra, Magnetic moments, Reaction mechanisms of the complexes. Assumptions of CFT: 1. The central Metal cation is surrounded by ligand which contain one or more lone pair of electrons. 2. The ionic ligand (F-, Cl- etc.) are regarded as point charges and neutral molecules (H2O, NH3 etc.) as point dipoles. 3. The electrons of ligand does not enter metal orbital. Thus there is no orbital overlap takes place. 4. The bonding between metal and ligand is purely electrostatic i.e. only ionic interaction. The approach taken uses classical potential energy equations that take into account the attractive and repulsive interactions between charged particles (that is, Coulomb's Law interactions). -

Interplay Between Gating and Block of Ligand-Gated Ion Channels
brain sciences Review Interplay between Gating and Block of Ligand-Gated Ion Channels Matthew B. Phillips 1,2, Aparna Nigam 1 and Jon W. Johnson 1,2,* 1 Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA; [email protected] (M.B.P.); [email protected] (A.N.) 2 Center for Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA * Correspondence: [email protected]; Tel.: +1-(412)-624-4295 Received: 27 October 2020; Accepted: 26 November 2020; Published: 1 December 2020 Abstract: Drugs that inhibit ion channel function by binding in the channel and preventing current flow, known as channel blockers, can be used as powerful tools for analysis of channel properties. Channel blockers are used to probe both the sophisticated structure and basic biophysical properties of ion channels. Gating, the mechanism that controls the opening and closing of ion channels, can be profoundly influenced by channel blocking drugs. Channel block and gating are reciprocally connected; gating controls access of channel blockers to their binding sites, and channel-blocking drugs can have profound and diverse effects on the rates of gating transitions and on the stability of channel open and closed states. This review synthesizes knowledge of the inherent intertwining of block and gating of excitatory ligand-gated ion channels, with a focus on the utility of channel blockers as analytic probes of ionotropic glutamate receptor channel function. Keywords: ligand-gated ion channel; channel block; channel gating; nicotinic acetylcholine receptor; ionotropic glutamate receptor; AMPA receptor; kainate receptor; NMDA receptor 1. Introduction Neuronal information processing depends on the distribution and properties of the ion channels found in neuronal membranes. -

Chapter 21 D-Metal Organometalloc Chemistry
Chapter 21 d-metal organometalloc chemistry Bonding Ligands Compounds Reactions Chapter 13 Organometallic Chemistry 13-1 Historical Background 13-2 Organic Ligands and Nomenclature 13-3 The 18-Electron Rule 13-4 Ligands in Organometallic Chemistry 13-5 Bonding Between Metal Atoms and Organic π Systems 13-6 Complexes Containing M-C, M=C, and M≡C Bonds 13-7 Spectral Analysis and Characterization of Organometallic Complexes “Inorganic Chemistry” Third Ed. Gary L. Miessler, Donald A. Tarr, 2004, Pearson Prentice Hall http://en.wikipedia.org/wiki/Expedia 13-1 Historical Background Sandwich compounds Cluster compounds 13-1 Historical Background Other examples of organometallic compounds 13-1 Historical Background Organometallic Compound Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Organometallic chemistry combines aspects of inorganic chemistry and organic chemistry. Organometallic compounds find practical use in stoichiometric and catalytically active compounds. Electron counting is key in understanding organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities of organometallic compounds. Organometallic compounds which have 18 electrons (filled s, p, and d orbitals) are relatively stable. This suggests the compound is isolable, but it can result in the compound being inert. 13-1 Historical Background In attempt to synthesize fulvalene Produced an orange solid (ferrocene) Discovery of ferrocene began the era of modern organometallic chemistry. Staggered -

Searching Coordination Compounds
CAS ONLINEB Available on STN Internationalm The Scientific & Technical Information Network SEARCHING COORDINATION COMPOUNDS December 1986 Chemical Abstracts Service A Division of the American Chemical Society 2540 Olentangy River Road P.O. Box 3012 Columbus, OH 43210 Copyright O 1986 American Chemical Society Quoting or copying of material from this publication for educational purposes is encouraged. providing acknowledgment is made of the source of such material. SEARCHING COORDINATION COMPOUNDS prepared by Adrienne W. Kozlowski Professor of Chemistry Central Connecticut State University while on sabbatical leave as a Visiting Educator, Chemical Abstracts Service Table of Contents Topic PKEFACE ............................s.~........................ 1 CHAPTER 1: INTRODUCTION TO SEARCHING IN CAS ONLINE ............... 1 What is Substructure Searching? ............................... 1 The Basic Commands .............................................. 2 CHAPTEK 2: INTKOOUCTION TO COORDINATION COPPOUNDS ................ 5 Definitions and Terminology ..................................... 5 Ligand Characteristics.......................................... 6 Metal Characteristics .................................... ... 8 CHAPTEK 3: STKUCTUKING AND REGISTKATION POLICIES FOR COORDINATION COMPOUNDS .............................................11 Policies for Structuring Coordination Compounds ................. Ligands .................................................... Ligand Structures........................................... Metal-Ligand -

Complexation
FACULTY OF PHARMACEUTICAL SCIENCES, RAMAUNIVERSITY, KANPUR B.PHARM 3rd SEM PHYSICAL PHARMACEUTICS-I BP302T MR. PEEYUSH Assistant professor Rama university, kanpur Complexation Overview Classification Introduction Metal ion complexes Organic Complexes Inclusion Complexes Methods of Analysis Method of Continuous Variation PH Titration Distribution Method Solubility Method Spectroscopy Learning Objectives 1. Define the three classes of complexes with pharmaceutically relevant examples. 2. Describe chelates, their physically properties, and what differentiates them from organic molecular complexes. 3. Describe the types of forces that hold together organic molecular complexes with examples. 4. Describe the forces in polymer–drug complexes used for drug delivery. 5. Discuss the pharmaceutical applications of cyclodextrins. 6. Describe the methods of analysis of complexes and determine their stoichiometric ratios and stability constants. Classification Introduction Metal ion complexes Organic Complexes Inclusion Complexes INTRODUCTION Complexes are compounds that result from donor–acceptor mechanisms between two or more chemical species. Complexes can be divided broadly into three classes depending the type of the acceptor substance: 1. Metal ion complexes 2. Organic molecular complexes 3. Inclusion complexes Intermolecular forces involved in the formation of complexes: 1. Van der Waals forces. 2. Hydrogen bonds (important in molecular complexes). 3. Coordinate covalence (important in metal complexes). 4. Charge transfer. 5. Hydrophobic interaction. Introduction Types of Complexes Metal Ion Complexes A. Inorganic type B. Chelates C. Olefin type D. Aromatic type II. Organic Molecular Complexes A. Quinhydrone type B. Picric acid type C. Caffeine and other drug complexes D. Polymer type III. Inclusion Compounds A. Channel lattice type B. Layer type C. Clathrates D. Monomolecular type E. -

Physical Organic Chemistry
PHYSICAL ORGANIC CHEMISTRY Yu-Tai Tao (陶雨台) Tel: (02)27898580 E-mail: [email protected] Website:http://www.sinica.edu.tw/~ytt Textbook: “Perspective on Structure and Mechanism in Organic Chemistry” by F. A. Corroll, 1998, Brooks/Cole Publishing Company References: 1. “Modern Physical Organic Chemistry” by E. V. Anslyn and D. A. Dougherty, 2005, University Science Books. Grading: One midterm (45%) one final exam (45%) and 4 quizzes (10%) homeworks Chap.1 Review of Concepts in Organic Chemistry § Quantum number and atomic orbitals Atomic orbital wavefunctions are associated with four quantum numbers: principle q. n. (n=1,2,3), azimuthal q.n. (m= 0,1,2,3 or s,p,d,f,..magnetic q. n. (for p, -1, 0, 1; for d, -2, -1, 0, 1, 2. electron spin q. n. =1/2, -1/2. § Molecular dimensions Atomic radius ionic radius, ri:size of electron cloud around an ion. covalent radius, rc:half of the distance between two atoms of same element bond to each other. van der Waal radius, rvdw:the effective size of atomic cloud around a covalently bonded atoms. - Cl Cl2 CH3Cl Bond length measures the distance between nucleus (or the local centers of electron density). Bond angle measures the angle between lines connecting different nucleus. Molecular volume and surface area can be the sum of atomic volume (or group volume) and surface area. Principle of additivity (group increment) Physical basis of additivity law: the forces between atoms in the same molecule or different molecules are very “short range”. Theoretical determination of molecular size:depending on the boundary condition. -
High Spin Or Low Spin?
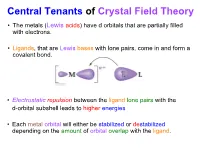
Central Tenants of Crystal Field Theory • The metals (Lewis acids) have d orbitals that are partially filled with electrons. • Ligands, that are Lewis bases with lone pairs, come in and form a covalent bond. • Electrostatic repulsion between the ligand lone pairs with the d-orbital subshell leads to higher energies • Each metal orbital will either be stabilized or destabilized depending on the amount of orbital overlap with the ligand. CFT (Octahedron) (dz2, dx2-y2) Orbitals point directly at ligands eg stronger repulsion dx2y2 E dz2 higher energy (dxy, dyz, dxz) Orbitals point between ligands t2g dxy dyz dxz weaker repulsion lower energy Energy Levels of d-Orbitals in an Octahedron • crystal field splitting energy = • The size of is determined by; metal (oxidation state; row of metal, for 5d > 4d >> 3d) ligand (spectrochemical series) Spectrochemical Series eg eg t2g t2g 3+ [Cr(H2O)6] Greater I < Br < Cl < F < OH < H2O < NH3 < en < NO2 < CN < CO Small Δ Large Δ Weak Field Strong Field Weak M-L interactions Strong M-L interactions How Do the Electrons Go In? • Hund’s rule: one electron each in lowest energy orbitals first • what happens from d4 to d7? d6 Crystal Field Spin Pairing Splitting vs. Δ P High Spin vs. Low Spin Co3+ (d6) High Spin Low Spin Δ < P Δ > P High Spin vs. Low Spin Configurations High Spin vs. Low Spin Configurations high spin low spin d4 3d metal 3d metal weak field strong field ligand d5 ligand 4d & 5d 6 d always d7 What About Other Geometries? linear (CN = 2) z y x tetrahedral (CN = 4) z y x square planar (CN = 4) z how do the electrons on the d orbitals y interact with the ligands in these cases? x Tetrahedral vs. -

Intriguing Role of Water in Protein-Ligand Binding Studied by Neutron Crystallography on Trypsin Complexes
ARTICLE DOI: 10.1038/s41467-018-05769-2 OPEN Intriguing role of water in protein-ligand binding studied by neutron crystallography on trypsin complexes Johannes Schiebel1,2, Roberto Gaspari2, Tobias Wulsdorf1, Khang Ngo1, Christian Sohn1, Tobias E. Schrader 3, Andrea Cavalli2, Andreas Ostermann 4, Andreas Heine1 & Gerhard Klebe1 fi 1234567890():,; Hydrogen bonds are key interactions determining protein-ligand binding af nity and therefore fundamental to any biological process. Unfortunately, explicit structural information about hydrogen positions and thus H-bonds in protein-ligand complexes is extremely rare and similarly the important role of water during binding remains poorly understood. Here, we report on neutron structures of trypsin determined at very high resolutions ≤1.5 Å in uncomplexed and inhibited state complemented by X-ray and thermodynamic data and computer simulations. Our structures show the precise geometry of H-bonds between protein and the inhibitors N-amidinopiperidine and benzamidine along with the dynamics of the residual solvation pattern. Prior to binding, the ligand-free binding pocket is occupied by water molecules characterized by a paucity of H-bonds and high mobility resulting in an imperfect hydration of the critical residue Asp189. This phenomenon likely constitutes a key factor fueling ligand binding via water displacement and helps improving our current view on water influencing protein–ligand recognition. 1 Institut für Pharmazeutische Chemie, Philipps-Universität Marburg, Marbacher Weg 6, 35032 Marburg, Germany. 2 Computational Sciences, Istituto Italiano di Tecnologia, Via Morego 30, 16163 Genova, Italy. 3 Jülich Centre for Neutron Science at Heinz Maier-Leibnitz Zentrum, Forschungszentrum Jülich, Lichtenbergstraße 1, 85748 Garching, Germany. 4 Heinz Maier-Leibnitz Zentrum, Technische Universität München, Lichtenbergstraße 1, 85748 Garching, Germany. -

Ligand-Based Carbon-Nitrogen Bond Forming Reactions of Metal
Ligand-Based CarbonÀNitrogen Bond Forming Reactions of Metal Dinitrosyl Complexes with Alkenes and Their Application to CÀH Bond Functionalization CHEN ZHAO, MARK R. CRIMMIN, F. DEAN TOSTE,* AND ROBERT G. BERGMAN* Department of Chemistry, University of California, Berkeley, Berkeley, California, 94720-1460, United States RECEIVED ON JULY 29, 2013 CONSPECTUS ver the past few decades, researchers have made substan- O tial progress in the development of transition metal com- plexes that activate and functionalize CÀH bonds. For the most part, chemists have focused on aliphatic and aromatic CÀH bonds and have put less effort into complexes that activate and function- alize vinylic CÀH bonds. Our groups have recently developed a novel method to functionalize vinylic CÀH bonds that takes advantage of the unique ligand-based reactivity of a rare class of metal dinitrosyl complexes. In this Account, we compare and discuss the chemistry of cobalt and ruthenium dinitrosyl complexes, emphasizing alkene binding, CÀH functionalization, and catalysis. Initially discovered in the early 1970s by Brunner and studied more extensively in the 1980s by the Bergman group, the cyclopentadienylcobalt dinitrosyl complex CpCo(NO)2 reacts rever- sibly with alkenes to give, in many cases, stable and isolable cobalt dinitrosoalkane complexes. More recently, we found that treatment with strong bases, such as lithium hexamethyldisilazide, Verkade's base, and phosphazene bases, deprotonates these complexes and renders them nucleophilic at the carbon r to the nitroso group. This conjugate anion of metal dinitrosoalkanes can participate in conjugate addition to Michael acceptors to form new carbonÀcarbon bonds. These functionalized cobalt complexes can further react through alkene exchange to furnish the overall vinylic CÀH functionalized organic product. -

Lecture 2--2016
Chem 462 Lecture 2--2016 z [MLnXm] Classification of Ligands Metal Oxidation States Electron Counting Overview of Transition Metal Complexes 1.The coordinate covalent or dative bond applies in L:M 2.Lewis bases are called LIGANDS—all serve as σ-donors some are π-donors as well, and some are π-acceptors 3. Specific coordination number and geometries depend on metal and number of d-electrons 4. HSAB theory useful z [MLnXm] a) Hard bases stabilize high oxidation states b) Soft bases stabilize low oxidation states Classification of Ligands: The L, X, Z Approach Malcolm Green : The CBC Method (or Covalent Bond Classification) used extensively in organometallic chemistry. L ligands are derived from charge-neutral precursors: NH3, amines, N-heterocycles such as pyridine, PR3, CO, alkenes etc. X ligands are derived from anionic precursors: halides, hydroxide, alkoxide alkyls—species that are one-electron neutral ligands, but two electron 4- donors as anionic ligands. EDTA is classified as an L2X4 ligand, features four anions and two neutral donor sites. C5H5 is classified an L2X ligand. Z ligands are RARE. They accept two electrons from the metal center. They donate none. The “ligand” is a Lewis Acid that accepts electrons rather than the Lewis Bases of the X and L ligands that donate electrons. Here, z = charge on the complex unit. z [MLnXm] octahedral Square pyramidal Trigonal bi- pyramidal Tetrahedral Range of Oxidation States in 3d Transition Metals Summary: Classification of Ligands, II: type of donor orbitals involved: σ; σ + π; σ + π*; π+ π* Ligands, Classification I, continued Electron Counting and the famous Sidgewick epiphany Two methods: 1) Neutral ligand (no ligand carries a charge—therefore X ligands are one electron donors, L ligands are 2 electron donors.) 2) Both L and X are 2-electron donor ligand (ionic method) Applying the Ionic or 2-electron donor method: Oxidative addition of H2 to chloro carbonyl bis triphenylphosphine Iridium(I) yields Chloro-dihydrido-carbonyl bis-triphenylphosphine Iridium(III). -

Metal Carbonyls
MODULE 1: METAL CARBONYLS Key words: Carbon monoxide; transition metal complexes; ligand substitution reactions; mononuclear carbonyls; dinuclear carbonyls; polynuclear carbonyls; catalytic activity; Monsanto process; Collman’s reagent; effective atomic number; 18-electron rule V. D. Bhatt / Selected topics in coordination chemistry / 2 MODULE 1: METAL CARBONYLS LECTURE #1 1. INTRODUCTION: Justus von Liebig attempted initial experiments on reaction of carbon monoxide with metals in 1834. However, it was demonstrated later that the compound he claimed to be potassium carbonyl was not a metal carbonyl at all. After the synthesis of [PtCl2(CO)2] and [PtCl2(CO)]2 reported by Schutzenberger (1868) followed by [Ni(CO)4] reported by Mond et al (1890), Hieber prepared numerous compounds containing metal and carbon monoxide. Compounds having at least one bond between carbon and metal are known as organometallic compounds. Metal carbonyls are the transition metal complexes of carbon monoxide containing metal-carbon bond. Lone pair of electrons are available on both carbon and oxygen atoms of carbon monoxide ligand. However, as the carbon atoms donate electrons to the metal, these complexes are named as carbonyls. A variety of such complexes such as mono nuclear, poly nuclear, homoleptic and mixed ligand are known. These compounds are widely studied due to industrial importance, catalytic properties and structural interest. V. D. Bhatt / Selected topics in coordination chemistry / 3 Carbon monoxide is one of the most important π- acceptor ligand. Because of its π- acidity, carbon monoxide can stabilize zero formal oxidation state of metals in carbonyl complexes. 2. SYNTHESIS OF METAL CARBONYLS Following are some of the general methods of preparation of metal carbonyls.