Clinical Profile and Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
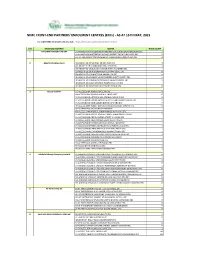
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (Ercs) - AS at 15TH MAY, 2021
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (ERCs) - AS AT 15TH MAY, 2021 For other NIMC enrolment centres, visit: https://nimc.gov.ng/nimc-enrolment-centres/ S/N FRONTEND PARTNER CENTER NODE COUNT 1 AA & MM MASTER FLAG ENT LA-AA AND MM MATSERFLAG AGBABIAKA STR ILOGBO EREMI BADAGRY ERC 1 LA-AA AND MM MATSERFLAG AGUMO MARKET OKOAFO BADAGRY ERC 0 OG-AA AND MM MATSERFLAG BAALE COMPOUND KOFEDOTI LGA ERC 0 2 Abuchi Ed.Ogbuju & Co AB-ABUCHI-ED ST MICHAEL RD ABA ABIA ERC 2 AN-ABUCHI-ED BUILDING MATERIAL OGIDI ERC 2 AN-ABUCHI-ED OGBUJU ZIK AVENUE AWKA ANAMBRA ERC 1 EB-ABUCHI-ED ENUGU BABAKALIKI EXP WAY ISIEKE ERC 0 EN-ABUCHI-ED UDUMA TOWN ANINRI LGA ERC 0 IM-ABUCHI-ED MBAKWE SQUARE ISIOKPO IDEATO NORTH ERC 1 IM-ABUCHI-ED UGBA AFOR OBOHIA RD AHIAZU MBAISE ERC 1 IM-ABUCHI-ED UGBA AMAIFEKE TOWN ORLU LGA ERC 1 IM-ABUCHI-ED UMUNEKE NGOR NGOR OKPALA ERC 0 3 Access Bank Plc DT-ACCESS BANK WARRI SAPELE RD ERC 0 EN-ACCESS BANK GARDEN AVENUE ENUGU ERC 0 FC-ACCESS BANK ADETOKUNBO ADEMOLA WUSE II ERC 0 FC-ACCESS BANK LADOKE AKINTOLA BOULEVARD GARKI II ABUJA ERC 1 FC-ACCESS BANK MOHAMMED BUHARI WAY CBD ERC 0 IM-ACCESS BANK WAAST AVENUE IKENEGBU LAYOUT OWERRI ERC 0 KD-ACCESS BANK KACHIA RD KADUNA ERC 1 KN-ACCESS BANK MURTALA MOHAMMED WAY KANO ERC 1 LA-ACCESS BANK ACCESS TOWERS PRINCE ALABA ONIRU STR ERC 1 LA-ACCESS BANK ADEOLA ODEKU STREET VI LAGOS ERC 1 LA-ACCESS BANK ADETOKUNBO ADEMOLA STR VI ERC 1 LA-ACCESS BANK IKOTUN JUNCTION IKOTUN LAGOS ERC 1 LA-ACCESS BANK ITIRE LAWANSON RD SURULERE LAGOS ERC 1 LA-ACCESS BANK LAGOS ABEOKUTA EXP WAY AGEGE ERC 1 LA-ACCESS -

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

{L' 7 3-,\O Tfmeat Novem Ber 2002 [,:.R'nroini.;Tion
AFRICAN PROGRAMME, FOR ONCHOCE,RCIASIS CONTROL (APOC) Forth Year Technical RePort for Community Directed Treatment with Ivermectin (cDrI) Dambatta Bichi Lbasawa Begwei Shanono Ajingi Gwarzo Kabo Gaya Wudil Kiru Bebcii Rano Karaye Takai Sumaila Doguwa Lp L For Acu-,,, I r.. ..4+ Caoa5 C5D Kano State clE' . l.r Nigeria p il, /{l' 7 3-,\o tfmeat Novem ber 2002 [,:.r'nroini.;tion Tr-r,_ I pr_ A'"' EXECUTTVE SUMMARY Kano State is situated in the northern part of Nigeria. The State has 44local govemment areas out of which 18 are Meso endemic with few hyper-endemic foci. The State falls in the Sudan Savannah and Sahel zones. Howeyer, the endemic areas are generally located in the Sudan savannah. The Ivermectin Distribution Programme (IDP) is in the 7th treatment round in some of the LGAs while in the 6th treatment round in others. However, CDTI strategy started in 1999. The CDTI project is therefore implemented in 779 communities of the 18 APOC approved local governments. Mobilization of the community members was conducted in all the targeted communities. In addition to mobilization, the state officials conducted advocacy visits to all the endemic local government Areas. The Launching of the commencement of 2002 prograrnme, which was performed by His Excellency, the Deputy Governor of Kano State increased awareness and acceptance of Mectizan by the people in the State. Electronic media, town criers and CDDs were among the mobilization strategies adopted for community mobilization. Targeted Training and re-training of CDTI programme personnel was conducted at state, LGA, and community levels, for those that are new in the programme as well as those with training dfficulties. -

ESCMID Online Lecture Library @ by Author Outline
Completing The Endgame Global Polio Eradication ECCMID, April 27, 2015 ESCMID Online Lecture Library @ by author Outline • Progress toward wild poliovirus eradication • Withdrawal of type 2 Oral Polio Vaccine • Managing the long-term risks • Global program priorities in 2015 ESCMID Online Lecture Library @ by author Wild Poliovirus Eradication, 1988-2012 125 Polio Endemic countries 125 Polioto Endemic 3 endemiccountries countries 400 300 19882012 200 Polio cases (thousands) 100 Last type 2 polio in Last Polio Case in the world India 0 ESCMID Online Lecture Library 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 @ by author Beginning of the Endgame Success in India established strategic & scientific feasibility of poliovirus eradication Poliovirus Type 2 eradication raised concerns about continued use of tOPV ESCMID Online Lecture Library @ by author Endgame Plan Objectives , 2013-18 1. Poliovirus detection & interruption 2. OPV2 withdrawal, IPV introduction, immunization system strengthening 3. Facility Containment & Global Certification ESCMID Online Lecture Library 4. Legacy Planning @ by author Vaccine-derived polio outbreaks (cVDPVs) 2000-2014 >90% VDPV cases are type 2 (40% of Vaccine-associated polio is also type 2) Type 1 ESCMID Online LectureType 2Library Type 3 @ by author Justification for new endgame Polio eradication not feasible without removal of all poliovirus strains from populations ESCMID Online Lecture Library @ by author Interrupting Poliovirus Transmission ESCMID Online Lecture Library @ by author Not detected since Nov 2012 ESCMID Online Lecture Library @ by author Wild Poliovirus type 1 (WPV1) Cases, 2013 Country 2013 2014 Pakistan 93 174 Afghanistan 14 10 Nigeria 53 6 Somalia 194 5 Cameroon 4 5 Equatorial Guinea 0 5 Iraq 0 2 Syria 35 1 Endemic countries Infected countries Ethiopia 9 1 Kenya 14 0 ESCMID Online Lecture TotalLibrary 416 209 Israel = Env. -

Transcript of Hajiya Binta Abdulhamid Interviewer: Elisha Renne
GLOBAL FEMINISMS COMPARATIVE CASE STUDIES OF WOMEN’S ACTIVISM AND SCHOLARSHIP SITE: NIGERIA Transcript of Hajiya Binta Abdulhamid Interviewer: Elisha Renne Location: Kano, Nigeria Date: 31st January, 2020 University of Michigan Institute for Research on Women and Gender 1136 Lane Hall Ann Arbor, MI 48109-1290 Tel: (734) 764-9537 E-mail: [email protected] Website: http://www.umich.edu/~glblfem © Regents of the University of Michigan, 2017 Hajiya Binta Abdulhamid was born on March 20, 1965, in Kano, the capital of Kano State, in northern Nigeria. She attended primary school and girls’ secondary school in Kano and Kaduna State. Thereafter she attended classes at Bayero University in Kano, where she received a degree in Islamic Studies. While she initially wanted to be a journalist, in 1983 she was encouraged to take education courses at the tertiary level in order to serve as a principal in girls’ secondary schools in Kano State. While other women had served in this position, there has been no women from Kano State who had done so. She has subsequently worked under the Kano State Ministry of Education, serving as school principal in several girls’ secondary schools in Kano State. Her experiences as a principal and teacher in these schools has enabled her to support girl child education in the state and she has encouraged women students to complete their secondary school education and to continue on to postgraduate education. She sees herself as a woman-activist in her advocacy of women’s education and has been gratified to see many of her former students working as medical doctors, lawyers, and politicians. -
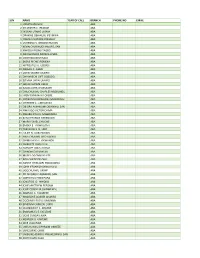
PROVISIONAL LIST.Pdf
S/N NAME YEAR OF CALL BRANCH PHONE NO EMAIL 1 JONATHAN FELIX ABA 2 SYLVESTER C. IFEAKOR ABA 3 NSIKAK UTANG IJIOMA ABA 4 ORAKWE OBIANUJU IFEYINWA ABA 5 OGUNJI CHIDOZIE KINGSLEY ABA 6 UCHENNA V. OBODOCHUKWU ABA 7 KEVIN CHUKWUDI NWUFO, SAN ABA 8 NWOGU IFIONU TAGBO ABA 9 ANIAWONWA NJIDEKA LINDA ABA 10 UKOH NDUDIM ISAAC ABA 11 EKENE RICHIE IREMEKA ABA 12 HIPPOLITUS U. UDENSI ABA 13 ABIGAIL C. AGBAI ABA 14 UKPAI OKORIE UKAIRO ABA 15 ONYINYECHI GIFT OGBODO ABA 16 EZINMA UKPAI UKAIRO ABA 17 GRACE UZOME UKEJE ABA 18 AJUGA JOHN ONWUKWE ABA 19 ONUCHUKWU CHARLES NSOBUNDU ABA 20 IREM ENYINNAYA OKERE ABA 21 ONYEKACHI OKWUOSA MUKOSOLU ABA 22 CHINYERE C. UMEOJIAKA ABA 23 OBIORA AKINWUMI OBIANWU, SAN ABA 24 NWAUGO VICTOR CHIMA ABA 25 NWABUIKWU K. MGBEMENA ABA 26 KANU FRANCIS ONYEBUCHI ABA 27 MARK ISRAEL CHIJIOKE ABA 28 EMEKA E. AGWULONU ABA 29 TREASURE E. N. UDO ABA 30 JULIET N. UDECHUKWU ABA 31 AWA CHUKWU IKECHUKWU ABA 32 CHIMUANYA V. OKWANDU ABA 33 CHIBUEZE OWUALAH ABA 34 AMANZE LINUS ALOMA ABA 35 CHINONSO ONONUJU ABA 36 MABEL OGONNAYA EZE ABA 37 BOB CHIEDOZIE OGU ABA 38 DANDY CHIMAOBI NWOKONNA ABA 39 JOHN IFEANYICHUKWU KALU ABA 40 UGOCHUKWU UKIWE ABA 41 FELIX EGBULE AGBARIRI, SAN ABA 42 OMENIHU CHINWEUBA ABA 43 IGNATIUS O. NWOKO ABA 44 ICHIE MATTHEW EKEOMA ABA 45 ICHIE CORDELIA CHINWENDU ABA 46 NNAMDI G. NWABEKE ABA 47 NNAOCHIE ADAOBI ANANSO ABA 48 OGOJIAKU RUFUS UMUNNA ABA 49 EPHRAIM CHINEDU DURU ABA 50 UGONWANYI S. AHAIWE ABA 51 EMMANUEL E. -

Non-Timber Forest Products and Their Contribution to Households Income
Suleiman et al. Ecological Processes (2017) 6:23 DOI 10.1186/s13717-017-0090-8 RESEARCH Open Access Non-timber forest products and their contribution to households income around Falgore Game Reserve in Kano, Nigeria Muhammad Sabiu Suleiman1*, Vivian Oliver Wasonga1, Judith Syombua Mbau1, Aminu Suleiman2 and Yazan Ahmed Elhadi1 Abstract Introduction: In the recent decades, there has been growing interest in the contribution of non-timber forest products (NTFPs) to livelihoods, development, and poverty alleviation among the rural populace. This has been prompted by the fact that communities living adjacent to forest reserves rely to a great extent on the NTFPs for their livelihoods, and therefore any effort to conserve such resources should as a prerequisite understand how the host communities interact with them. Methods: Multistage sampling technique was used for the study. A representative sample of 400 households was used to explore the utilization of NTFPs and their contribution to households’ income in communities proximate to Falgore Game Reserve (FGR) in Kano State, Nigeria. Descriptive statistics and logistic regression analysis were used to analyze and summarize the data collected. Results: The findings reveal that communities proximate to FGR mostly rely on the reserve for firewood, medicinal herbs, fodder, and fruit nuts for household use and sales. Income from NTFPs accounts for 20–60% of the total income of most (68%) of the sampled households. The utilization of NTFPs was significantly influenced by age, sex, household size, main occupation, distance to forest and market. Conclusions: The findings suggest that NTFPs play an important role in supporting livelihoods, and therefore provide an important safety net for households throughout the year particularly during periods of hardship occasioned by drought. -

Zaria Geographer Vol. 23, No. 1, 2016 SPATIAL DISTRIBUTION OF
SPATIAL DISTRIBUTION OF PRIMARY HEALTH CARE FACILITIES IN KANO SOUTH SENATORIAL ZONE, KANO STATE, NIGERIA By Umar, N.K.1*, Musa, I.J.1, Hassan, M.1 and Obeka, S.S.2 1Department of Geography, Ahmadu Bello University, Zaria, Nigeria 2Department of Science Education, Ahmadu Bello University, Zaria, Nigeria *Corresponding Author’s Email: [email protected] ABSTRACT While PHC facilities are relatively uniformly distributed throughout Local Government Areas (LGAs) in Nigeria, the rural people tend to underuse the basic health services. Thus, the weakening of such public health services in terms of availability and the increasing demand of healthcare due to rapid population growth in the study area, might have placed PHC facilities in a deplorable situation. This study seek to analyze the spatial distribution of PHC facilities in some selected LGAs of Kano South Senatorial Zone, Kano State, Nigeria. These study locations were selected using systematic sampling technique. The study sites include Sumaila, Gaya, Ajingi and Rano LGAs. Data used for the study included: Administrative map and Google earth satellite imagery of Kano state, population data, coordinates of all the wards of the study area and the PHC facilities. Information on the number and addresses of the facilities were also used. These data were incorporated into ArcGIS 10.1 Software interface, mapped and analyzed using GIS Technique (Nearest Neighborhood) and descriptive statistics were also used. Results of the analysis showed that PHC facilities visually and descriptively within the study area, with Sumaila having the highest percentage (36.03%), while Rano had the least (12.61%). Furthermore, the Neighborhood analysis revealed similar distribution pattern of the facilities with less than 1% (0.01 significance level). -

Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria
Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria. May, 2015 i Table of Contents Acronyms ......................................................................................................................................................................v Foreword ......................................................................................................................................................................vi Acknowledgements ...............................................................................................................................................vii Executive Summary ..............................................................................................................................................viii 1.0 Background ............................................................................................................................................1 1.1 Introduction .................................................................................................................................................1 1.2 Objectives of the Mapping Project ..................................................................................................2 1.3 Justification for the Survey ..................................................................................................................2 2.0. Mapping Methodology ......................................................................................................................3 -

Nigeria: Current Locations of Internally Displaced Persons from Borno State (As of 30 April 2016)
Nigeria: Current locations of internally displaced persons from Borno state (as of 30 April 2016) 1.8 million NIGER Estimated number of people displaced from Borno state. (Source DTM Round IX and Joint UN Mission) Yusufari CHAD Machina Nguru Estimated number of internally displaced persons Karasuwa per Local Government Areas of origin* Bade Gubio Bama Bade Geidam 405,507 Kaura-Namoda Gwoza 250,772 Talata Makoda Dambatta Jakusko BORNO Mafara Konduga 157,802 Bungudu Bichi YOBE Tarmua Magumeri Damboa 126,104 Ungogo Gabasawa Maru Gezawa Zaki Gamawa Ngala 114,155 Gummi Tofa Ajingi Jere Mafa Tsafe Gwale Warawa Itas/Gadau Kabo Maiduguri Gubio 98,891 Nangere Fune Damaturu ZAMFARA Karaye Madobi WudilGaya Kaga Jama'are Katagum Potiskum 94,004 Bunkure Dawakin Kudu Damban Kaga Kiru Konduga Mafa 77,692 Gusau Rogo Kibiya Takali Shira Maru Giade Misau Kukawa 63,317 Markafi KANO Sumaila Hawul Ikara Darazo 53,054 Sabon-Gari Warji Nafada Damboa Monguno 45,173 Zaria Ningi Marte 41,291 Soba Kubau Doguwa Biu Chibok Madagali Dikwa 37,408 Igabi Ganjuwa Dukku Askira/Uba Askira/Uba 24,958 Kaduna North Michika Kwami Kwaya Maiduguri 9,296 Kaduna South BAUCHI Hawul Kirfi BayoKusar Hong Mubi North Jere 5,490 Kauru Gombe Yamaltu Chikun Bauchi Abadam 4,524 /Deba Shani Gombi Mubi South Bassa Akko Mobbar KADUNA Dass 4,286 Jos North GOMBE Alkaleri Magumeri 2,000 Zango-Kataf Tafawa-Balewa Billiri BalangaGuyuk Song Maiha Biu 895 Jos South Shomgom ADAMAWA CHAD Chibok Lamurde 861 Kagarko PLATEAU Kwaya/Kusar 181 Kanke Numan Gireri Bwari Yola South Guzamala 68 Bokkos Karin-Lamido -

Analysis of Human Capital Development Index in Kano State
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Developing Country Studies www.iiste.org ISSN 2224-607X (Paper) ISSN 2225-0565 (Online) Vol.7, No.5, 2017 Analysis of Human Capital Development Index in Kano State Dr. Nazifi Abdullahi Darma 1 Aigbedion I. Marvelous 1 Nafiu Bashir 2 Umar Abubakar kari 3 1.Department of Economics, University of Abuja 2.Department of Economics, Bayaro University Kano (BUK) 3.Department of Sociology, University of Abuja Abstract The study focuses on the analysis of human capital development index domesticated in Kano state, Nigeria. The main objective of this study is design human capital index and explores the contributors and inhibitors to the development and deployment of a healthy, educated and productive labour force in the state. The study covers the 44 local government areas and 122 wards of the State. Four key indicators were used for the design of human capital index in Kano State which are; level of education, health and wellness, workforce and employment and enabling environment. These indicators were analyzed using standardization method of ranking. From the index, some regions of the states have impressive and fair human capital index which are Northern Central, Eastern and North Western regions while some regions have poor ranking of human capital index in the State especially Far South, Western and Southern regions were most of the index were negative as per the defined indicators as captured by the level of education, health and wellness, workforce and employment and enabling environment. -

Preliminary Results from Direct-To-Facility Vaccine Deliveries in Kano, Nigeria
Preliminary results from direct-to-facility vaccine deliveries in Kano, Nigeria Muyi Aina Uchenna Igbokwe Solina Center for International Development and Research Rabiu Fagge Kano State Primary Health Care Management Board Presentation at the Health and Humanitarian Logistics Conference, Copenhagen June 8, 2017 CONFIDENTIAL AND PROPRIETARY Any use of this material without specific permission of Solina Health is strictly prohibited Content Background Methods Results Learnings Conclusion 2 General information on Kano state, Nigeria Kano at a glance Kano State has 44 LGAs ▪ 11 Million (2011) Population1 Makoda Kunchi Dambatta GDP per ▪ $1,288 USD Tsanyawa Bichi 2 Minjibir Gabasawa capita Dawakin Tofa Bagwai Ungogo Tofa Dala Gezawa Shanono Rimin Gado Tarauni Ajingi KumbotsoWarawa ▪ 0.44 Million Gwarzo Kabo No. of Madobi Dawakin Kudu Kura Gaya children <1 Wudil Karaye Bumkure Albasu Kiru Garun Mallam Garko Rogo Bebeji Rano ▪ 50 deaths per 1,000 Kibiya Takai Infant mortality rate3 children Tudun Wada Sumaila ▪ BCG: 27.5% Immun. ▪ DPT3: 18.9% coverage rate4 Doguwa ▪ Fully immunised: 13.2% ▪ 1,300 PHCs;, 1,142 0 50 100 Km Healthcare facilities providing RI services SOURCE: 1. City population statistics; 2. Canback Dangtel C-GIDD, 3. MDG 2014; 4. Nigeria Demographic and Health Survey, 2013 3 Historically, a weak vaccine supply chain system significantly contributed to poor immunization coverage rates in Kano Key supply chain bottlenecks resulted in …and contributed to the poor vaccination inadequate supply of vaccine for RI sessions… coverage in Kano and other northern states DPT3 coverage by state (NDHS 2013) 1 Inadequate cold chain and poor maintenance limiting vaccine availability at service points 2 Complex and ineffective distribution architecture causing frequent stock outs 3 Inadequate and ad-hoc funding for vaccine transportation across all levels 4 Faulty vaccine forecasting and allocation which did not adequately reflect demand 5 Weak data management systems resulting in ineffective management decision making 6 Lack of proper supportive supervision due to 1.