Early Aging Effect on the Function of the Human Central Olfactory System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantitative Analysis of Axon Collaterals of Single Pyramidal Cells
Yang et al. BMC Neurosci (2017) 18:25 DOI 10.1186/s12868-017-0342-7 BMC Neuroscience RESEARCH ARTICLE Open Access Quantitative analysis of axon collaterals of single pyramidal cells of the anterior piriform cortex of the guinea pig Junli Yang1,2*, Gerhard Litscher1,3* , Zhongren Sun1*, Qiang Tang1, Kiyoshi Kishi2, Satoko Oda2, Masaaki Takayanagi2, Zemin Sheng1,4, Yang Liu1, Wenhai Guo1, Ting Zhang1, Lu Wang1,3, Ingrid Gaischek3, Daniela Litscher3, Irmgard Th. Lippe5 and Masaru Kuroda2 Abstract Background: The role of the piriform cortex (PC) in olfactory information processing remains largely unknown. The anterior part of the piriform cortex (APC) has been the focus of cortical-level studies of olfactory coding, and asso- ciative processes have attracted considerable attention as an important part in odor discrimination and olfactory information processing. Associational connections of pyramidal cells in the guinea pig APC were studied by direct visualization of axons stained and quantitatively analyzed by intracellular biocytin injection in vivo. Results: The observations illustrated that axon collaterals of the individual cells were widely and spatially distrib- uted within the PC, and sometimes also showed a long associational projection to the olfactory bulb (OB). The data showed that long associational axons were both rostrally and caudally directed throughout the PC, and the intrinsic associational fibers of pyramidal cells in the APC are omnidirectional connections in the PC. Within the PC, associa- tional axons typically followed rather linear trajectories and irregular bouton distributions. Quantitative data of the axon collaterals of two pyramidal cells in the APC showed that the average length of axonal collaterals was 101 mm, out of which 79 mm (78% of total length) were distributed in the PC. -

On the Scent of Human Olfactory Orbitofrontal Cortex: Meta-Analysis and Comparison to Non-Human Primates
Brain Research Reviews 50 (2005) 287 – 304 www.elsevier.com/locate/brainresrev Review On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates Jay A. Gottfrieda,*, David H. Zaldb aDepartment of Neurology and the Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University Feinberg School of Medicine, 320 E. Superior St., Searle 11-453, Chicago, IL 60611, USA bDepartment of Psychology, Vanderbilt University, Nashville, TN 37240, USA Accepted 25 August 2005 Available online 6 October 2005 Abstract It is widely accepted that the orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay. While such findings are almost exclusively derived from animal data, recent cytoarchitectonic studies indicate a close anatomical correspondence between non-human primate and human OFC. Given this cross-species conservation of structure, it has generally been presumed that the olfactory projection area in human OFC occupies the same posterior portions of OFC as seen in non-human primates. This review questions this assumption by providing a critical survey of the localization of primate and human olfactory neocortex. Based on a meta-analysis of human functional neuroimaging studies, the region of human OFC showing the greatest olfactory responsivity appears substantially rostral and in a different cytoarchitectural area than the orbital olfactory regions as defined in the monkey. -

AN EXPERIMENTAL INVESTIGATION of the CONNEXIONS of the OLFACTORY TRACTS in the MONKEY by MARGARET MEYER and A
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.12.4.274 on 1 November 1949. Downloaded from J. Neurol. Neurosurg. Psychiat., 1949, 12, 274. AN EXPERIMENTAL INVESTIGATION OF THE CONNEXIONS OF THE OLFACTORY TRACTS IN THE MONKEY BY MARGARET MEYER and A. C. ALLISON From the Department ofAnatomy, University of Oxford The great expansion of the-cerebral cortex which bilateral degeneration of olfactory terminals appar- has taken place in higher primates has brought ently passing through the anterior limb of the about a considerable displacement of structures on anterior commissure. The present study has been the base of the telencephalon, and the precise undertaken to map out the connexions of the comparison of certain areas in this part of the olfactory bulb in the monkey's brain as precisely brain with those in lower mammals has been a as possible with the same silver technique. matter of some difficulty. This is true particularly Material and Methods of the olfactory areas which lie on the orbital aspect guest. Protected by copyright. of the frontal lobe and the adjacent part of the Three macaque monkeys (Macaca mulatta) and two this immature Guinea baboons (Papio papio) were used. temporal lobe. Although part of the brain The operative technique was similar in all cases: under in primates has been subjected to detailed cyto- nembutal anesthesia and with the usual aseptic pre- architectural and myelo-architectural examinations cautions a large right frontal bone flap was reflected; (Rose, 1927b, 1928; Beck, 1934, and others), the the frontal lobe of the hemisphere was carefully retraced, areas directly related to olfaction have never been and the olfactory peduncle, lying on the ventral surface, clearly defined. -

The Mitral Cells of the Bulb May Contain Atrophic Substance That Helps to Maintain the Survival of Olfactory Receptor Neurons (49)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by KhartoumSpace Structure and Central Connections Of PERIPHERAL OLFACTORY SYSTEM. Submitted in partial fulfillment for the degree of M. Sc. In human morphology. In the Anatomy Department U. of K. ASIM S. Y. Surij MBBS U. of K. 1980. Supervised by PROFESSOR: MOHEMED AHMED HASSAN A/ GALEEL. February, 2006. In the memory of, Professor Mustafa Hassan Badi, 1941-2004 A brilliant educationalist, And a scholarly scientist DEDICATION This work is dedicated to the lates, my mother; Ustaza Katera Ibraheem Sorij and my sister Ustaza Attiat Sidahmed. To my two daughters Tasneem and Toga. ACKNOWELDGEMENT. I would like to thank Professor Mohemed Ahmed Hassan, Head Anatomy Department, my supervisor in the Anatomy Department at the Medical School University of Khartoum, and Dr. Ammar Eltahir, ViceDean for academic affairs, Faculty of medicine, my co-supervisor whose careful remarks and assistance have produced this work in its present format. He availed his office and his own PCW, despite his tremendous obligations. His continuous interest in me, my work, and my family has made this work just possible. His secretaries were very helpful. Mr. Babiker Khalid subahi, senior chief scientific officer school of Pharmacy has availed his PCW and technical expertise all throughout this work. In the medical illustrations Department Mr. Babiker Othman has helped with the photomicrographs. To all these kind Sudanese people goes my gratitude. ABSTRACT Olfaction, the sense of smell is an old sensory modality; in fact the whole olfactory system belongs to a phylogenetically old part of the neuraxis, the paleocortex. -

Olfactory Cortex Development
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Review | Development Development and organization of the evolutionary conserved three-layered olfactory cortex Olfactory cortex development Esther Klingler Department of Basic Neuroscience, University of Geneva, Geneva, Switzerland. DOI: 10.1523/ENEURO.0193-16.2016 Received: 6 July 2016 Revised: 11 November 2016 Accepted: 8 December 2016 Published: 20 January 2017 Author Contributions: EK designed and wrote the paper. Conflict of Interest: Authors report no conflict of interest. Correspondence should be addressed to Esther Klingler, Jabaudon Lab, Department of Basic Neuroscience, University of Geneva, CMU, 1, rue Michel Servet, 1206 Geneva – Switzerland, E-mail: [email protected] Cite as: eNeuro 2017; 10.1523/ENEURO.0193-16.2016 Alerts: Sign up at eneuro.org/alerts to receive customized email alerts when the fully formatted version of this article is published. Accepted manuscripts are peer-reviewed but have not been through the copyediting, formatting, or proofreading process. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. Copyright © 2017 the authors ϭ Manuscript Title Page Ϯ ϯ ϰ 1. Manuscript Title ϱ Ǧ Ǥ ϲ ϳ 2. Abbreviated Title ϴ ϵ ϭϬ 3. List of all Author Names and Affiliations ϭϭ ǣ ǡ ǡ ǡǤ ϭϮ ϭϯ 4. Author Contributions ϭϰ Ǥ ϭϱ ϭϲ 5. Correspondence should be addressed to ϭϳ ǡǤ̷Ǥ ϭϴ ΪͶͳʹʹ͵ͻͶͳ ϭϵ ϮϬ ǡ ǡ Ϯϭ ͳǡ ϮϮ ͳʹͲ Ǧ Ϯϯ Ϯϰ 6. -

Preprocessing of Emotional Visual Information in the Human Piriform Cortex Received: 10 January 2017 Patrick Schulze1, Anne-Kathrin Bestgen2, Robert K
www.nature.com/scientificreports OPEN Preprocessing of emotional visual information in the human piriform cortex Received: 10 January 2017 Patrick Schulze1, Anne-Kathrin Bestgen2, Robert K. Lech1, Lars Kuchinke3 & Boris Suchan1 Accepted: 25 July 2017 This study examines the processing of visual information by the olfactory system in humans. Recent Published: xx xx xxxx data point to the processing of visual stimuli by the piriform cortex, a region mainly known as part of the primary olfactory cortex. Moreover, the piriform cortex generates predictive templates of olfactory stimuli to facilitate olfactory processing. This study fills the gap relating to the question whether this region is also capable of preprocessing emotional visual information. To gain insight into the preprocessing and transfer of emotional visual information into olfactory processing, we recorded hemodynamic responses during affective priming using functional magnetic resonance imaging (fMRI). Odors of different valence (pleasant, neutral and unpleasant) were primed by images of emotional facial expressions (happy, neutral and disgust). Our findings are the first to demonstrate that the piriform cortex preprocesses emotional visual information prior to any olfactory stimulation and that the emotional connotation of this preprocessing is subsequently transferred and integrated into an extended olfactory network for olfactory processing. The interaction between olfactory and visual information contributes to an effective perception of odors. For example, odor identification is improved by additional verbal information1 and odor detection is enhanced by the presentation of an odor-source congruent picture (e.g. a picture of oranges, when an orange odor is presented)2. Thus, the olfactory system strongly benefits from information provided by the visual system. -

Lecture 14 --Olfaction.Pdf
14 Olfaction ClickChapter to edit 14 MasterOlfaction title style • Olfactory Physiology • Neurophysiology of Olfaction • From Chemicals to Smells • Olfactory Psychophysics, Identification, and Adaptation • Olfactory Hedonics • Associative Learning and Emotion: Neuroanatomical and Evolutionary Considerations ClickIntroduction to edit Master title style Olfaction: The sense of smell Gustation: The sense of taste ClickOlfactory to edit Physiology Master title style Odor: The translation of a chemical stimulus into a smell sensation. Odorant: A molecule that is defined by its physiochemical characteristics, which are capable of being translated by the nervous system into the perception of smell. To be smelled, odorants must be: • Volatile (able to float through the air) • Small • Hydrophobic (repellent to water) Figure 14.1 Odorants ClickOlfactory to edit Physiology Master title style The human olfactory apparatus • Unlike other senses, smell is tacked onto an organ with another purpose— the nose. Primary purpose—to filter, warm, and humidify air we breathe . Nose contains small ridges, olfactory cleft, and olfactory epithelium ClickOlfactory to edit Physiology Master title style The human olfactory apparatus (continued) • Olfactory cleft: A narrow space at the back of the nose into which air flows, where the main olfactory epithelium is located. • Olfactory epithelium: A secretory mucous membrane in the human nose whose primary function is to detect odorants in inhaled air. Figure 14.2 The nose ClickOlfactory to edit Physiology Master title style Olfactory epithelium: The “retina” of the nose • Three types of cells . Supporting cells: Provide metabolic and physical support for the olfactory sensory neurons. Basal cells: Precursor cells to olfactory sensory neurons. Olfactory sensory neurons (OSNs): The main cell type in the olfactory epithelium. -

Characterizing Functional Pathways of the Human Olfactory System Guangyu Zhou1*, Gregory Lane1, Shiloh L Cooper1, Thorsten Kahnt1,2, Christina Zelano1*
RESEARCH ARTICLE Characterizing functional pathways of the human olfactory system Guangyu Zhou1*, Gregory Lane1, Shiloh L Cooper1, Thorsten Kahnt1,2, Christina Zelano1* 1Department of Neurology, Feinberg School of Medicine, Northwestern University, Chicago, United States; 2Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, Evanston, United States Abstract The central processing pathways of the human olfactory system are not fully understood. The olfactory bulb projects directly to a number of cortical brain structures, but the distinct networks formed by projections from each of these structures to the rest of the brain have not been well-defined. Here, we used functional magnetic resonance imaging and k-means clustering to parcellate human primary olfactory cortex into clusters based on whole-brain functional connectivity patterns. Resulting clusters accurately corresponded to anterior olfactory nucleus, olfactory tubercle, and frontal and temporal piriform cortices, suggesting dissociable whole-brain networks formed by the subregions of primary olfactory cortex. This result was replicated in an independent data set. We then characterized the unique functional connectivity profiles of each subregion, producing a map of the large-scale processing pathways of the human olfactory system. These results provide insight into the functional and anatomical organization of the human olfactory system. DOI: https://doi.org/10.7554/eLife.47177.001 Introduction *For correspondence: The human sense of smell serves a variety of important functions in everyday life (Bushdid et al., [email protected] (GZ); 2014; Devanand et al., 2015; McGann, 2017). It is used to monitor the safety of inhaled air [email protected] (CZ) (Pence et al., 2014) and edibility of food (Yeomans, 2006). -

Olfactory Nerve.Pdf
OLFACTORY NERVE Introduction ► First cranial nerve ► One of the two cranial nerves which doesn’t course through the posterior fossa ► Only neurons which can regenerate (basal cells) ► Only sensation which is not processed in the thalamus directly Overview of olfactory system ► Sensory system used for smell ► Represents one of the oldest sensory modalities in the phylogenetic history of mammals ► Less developed in humans than in other mammals such as rodents. As a chemical sensor, the olfactory system detects food and influences social and sexual behavior. ► 2 distinct parts- § Main- for volatile air-borne stimuli § Accessory- for fluid-phase stimuli ► Often spoken along with the gustatory system as the chemosensory senses ► Mechanism-Peripheral and central Peripheral component ► External stimulus( odour) ► Olfactory receptors in olfactory epithelium ► Transduction of receptor activation into electric signals ► Signals travel along the olfactory nerves (part of peripheral) ► End in olfactory bulb ( part of central) Central component ► Olfactory bulb ► Medial and lateral olfactory striae ► Terminal areas ► First order- Bipolar sensory cells in the olfactory epithelium ► Second order- Mitral and tufted cells in the olfactory glomeruli ► Third order- Neurons in the olfactory cortex Olfactory epithelium ► Specialised epithelial tissue inside the nasal cavity that is involved in smell ► It is about 1 cm2 on each side and lies in the roof of nasal cavity around 7 cm above and behind the nostrils ► Part of the olfactory system directly responsible for detecting odors ► 3 types of cells- olfactory, supporting, basal cells Cells ► Olfactory cells- Bipolar cells which congregrate to form the olfactory nerve ► Supporting/ sustentacular cells- metabolic and physical support to olfactory cells ► Basal cells- STEM CELLS capable of differentiating into either of the two. -
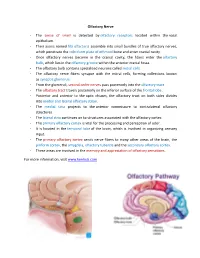
Olfactory Nerve
Olfactory Nerve - The sense of smell is detected by olfactory receptors located within the nasal epithelium. - Their axons named fila olfactoria assemble into small bundles of true olfactory nerves, which penetrate the cribriform plate of ethmoid bone and enter cranial cavity. - Once olfactory nerves become in the cranial cavity, the fibers enter the olfactory bulb, which lies in the olfactory groove within the anterior cranial fossa. - The olfactory bulb contains specialized neurons called mitral cells. - The olfactory nerve fibers synapse with the mitral cells, forming collections known as synaptic glomeruli. - From the glomeruli, second order nerves pass posteriorly into the olfactory tract. - The olfactory tract travels posteriorly on the inferior surface of the frontal lobe. - Posterior and anterior to the optic chiasm, the olfactory tract on both sides divides into medial and lateral olfactory striae. - The medial stria projects to the anterior commissure to contralateral olfactory structures. - The lateral stria continues on to structures associated with the olfactory cortex. - The primary olfactory cortex is vital for the processing and perception of odor. - It is located in the temporal lobe of the brain, which is involved in organizing sensory input. - The primary olfactory cortex sends nerve fibers to many other areas of the brain, the piriform cortex, the amygdala, olfactory tubercle and the secondary olfactory cortex. - These areas are involved in the memory and appreciation of olfactory sensations. For more information, visit www.kenhub.com . -

PTSD and Olfaction
PTSD and Olfaction The Aetiology of PTSD and the Bidirectional Relationship between Trauma and Sensitisation. Thesis submitted for the degree of Doctor of Philosophy By William Graham Hough February 2014 The University of Adelaide, Australia Discipline of Psychiatry Centre for Traumatic Stress Studies Faculty of Health Science TABLE OF CONTENTS LIST OF TABLES .................................................................................................. x LIST OF FIGURES .............................................................................................. xii ABSTRACT ......................................................................................................... xiii DECLARATION .................................................................................................. xv ACKNOWLEDGEMENTS ................................................................................. xvi CHAPTER 1: INTRODUCTION ........................................................................... 1 Description of the Problem ................................................................................. 1 Importance of the Study ...................................................................................... 1 Methods of Study ................................................................................................ 5 Significant Findings ............................................................................................ 6 Implications ....................................................................................................... -

Olfactory System and Emotion: Common Substrates
European Annals of Otorhinolaryngology, Head and Neck diseases (2011) 128, 18—23 View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector REVIEW OF THE LITERATURE Olfactory system and emotion: Common substrates Y. Soudry a, C. Lemogne a,b,c, D. Malinvaud d, S.-M. Consoli a,b,c, P. Bonfils b,d,∗,e a Service de psychologie clinique et de liaison, de psychiatrie, hôpital européen Georges-Pompidou, Assistance publique—Hôpitaux de Paris, 75015 Paris, France b Faculté de médecine Paris-Descartes, université Paris-V, 75006 Paris, France c CNRS UMR 7593, hôpital de la Pitié-Salpêtrière, 75013 Paris, France d Département d’ORL et de chirurgie cervico-faciale, hôpital européen Georges-Pompidou, Assistance publique—Hôpitaux de Paris, 20, rue Leblanc, 75015 Paris cedex 15, France e Laboratoire « centre d’études de la sensori-motricité » (CESEM), CNRS UMR 8194, 75006 Paris, France Available online 11 January 2011 KEYWORDS Summary Olfaction; The aim of the review: A large number of studies suggest a close relationship between olfactory Emotion; and affective information processing. Odors can modulate mood, cognition, and behavior. The Piriform cortex; aim of this article is to summarize the comparative anatomy of central olfactory pathways and Entorhinal cortex; centers involved in emotional analysis, in order to shed light on the relationship between the Orbitofrontal cortex two systems. Anatomy of the olfactory system: Odorant contact with the primary olfactory neurons is the starting point of olfactory transduction. The glomerulus of the olfactory bulb is the only relay between the peripheral and central olfactory system.