Olfactory System and Emotion: Common Substrates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distinct Representations of Olfactory Information in Different Cortical Centres
LETTER doi:10.1038/nature09868 Distinct representations of olfactory information in different cortical centres Dara L. Sosulski1, Maria Lissitsyna Bloom1{, Tyler Cutforth1{, Richard Axel1 & Sandeep Robert Datta1{ Sensory information is transmitted to the brain where it must be behaviours, but is unlikely to specify innate behaviours. Rather, innate processed to translate stimulus features into appropriate beha- olfactory behaviours are likely to result from the activation of genetically vioural output. In the olfactory system, distributed neural activity determined, stereotyped neural circuits. We have therefore developed a in the nose is converted into a segregated map in the olfactory strategy to trace the projections from identified glomeruli in the olfactory bulb1–3. Here we investigate how this ordered representation is bulb to higher olfactory cortical centres. transformed in higher olfactory centres in mice. We have Mitral and tufted cells that innervate a single glomerulus were developed a tracing strategy to define the neural circuits that labelled by electroporation of tetramethylrhodamine (TMR)-dextran convey information from individual glomeruli in the olfactory under the guidance of a two-photon microscope. This technique labels bulb to the piriform cortex and the cortical amygdala. The spatial mitral and tufted cells that innervate a single glomerulus and is suffi- order in the bulb is discarded in the piriform cortex; axons from ciently robust to allow the identification of axon termini within mul- individual glomeruli project diffusely to the piriform without tiple higher order olfactory centres (Figs 1a–c, 2 and Supplementary apparent spatial preference. In the cortical amygdala, we observe Figs 1–4). Labelling of glomeruli in the olfactory bulbs of mice that broad patches of projections that are spatially stereotyped for indi- express GFP under the control of specific odorant receptor promoters vidual glomeruli. -

Early Aging Effect on the Function of the Human Central Olfactory System
Journals of Gerontology: Biological Sciences cite as: J Gerontol A Biol Sci Med Sci, 2017, Vol. 72, No. 8, 1007–1014 doi:10.1093/gerona/glw104 Advance Access publication June 11, 2016 Original Article Early Aging Effect on the Function of the Human Central Downloaded from https://academic.oup.com/biomedgerontology/article/72/8/1007/2629931 by guest on 27 September 2021 Olfactory System Choice Editor’s Jianli Wang,1 Xiaoyu Sun,1 and Qing X. Yang2 1Department of Radiology, Pennsylvania State University College of Medicine, Hershey. 2Departments of Radiology and Neurosurgery, Pennsylvania State University College of Medicine, Hershey. Address correspondence to Jianli Wang, MD, PhD, Department of Radiology, Pennsylvania State University College of Medicine, Hershey, PA 17033. E-mail: [email protected] Received November 23, 2015; Accepted April 27, 2016 Decision Editor: Rafael de Cabo, PhD Abstract During normal aging process, the smell function declines significantly, starting from the sixth decade of age. While it has been shown that activity in the central olfactory system of seniors responding to odor stimulation is significantly less than that of young people, no information of the aging effect on the functions of this system during normal adulthood and early aging has been gathered. In this study, we used functional magnetic resonance imaging to investigate the olfaction-related brain activity in the central olfactory structures of 43 healthy adult volunteers aged from 22 to 64 years. The participants’ smell identification function was negatively correlated with age (r = −.32, p = .037). Significant negative correlation was observed between age and the olfaction-related activities in the bilateral dorsolateral prefrontal cortex, left insular cortex, and left orbitofrontal cortex (p < .001, corrected with cluster size ≥28 voxels). -

Quantitative Analysis of Axon Collaterals of Single Pyramidal Cells
Yang et al. BMC Neurosci (2017) 18:25 DOI 10.1186/s12868-017-0342-7 BMC Neuroscience RESEARCH ARTICLE Open Access Quantitative analysis of axon collaterals of single pyramidal cells of the anterior piriform cortex of the guinea pig Junli Yang1,2*, Gerhard Litscher1,3* , Zhongren Sun1*, Qiang Tang1, Kiyoshi Kishi2, Satoko Oda2, Masaaki Takayanagi2, Zemin Sheng1,4, Yang Liu1, Wenhai Guo1, Ting Zhang1, Lu Wang1,3, Ingrid Gaischek3, Daniela Litscher3, Irmgard Th. Lippe5 and Masaru Kuroda2 Abstract Background: The role of the piriform cortex (PC) in olfactory information processing remains largely unknown. The anterior part of the piriform cortex (APC) has been the focus of cortical-level studies of olfactory coding, and asso- ciative processes have attracted considerable attention as an important part in odor discrimination and olfactory information processing. Associational connections of pyramidal cells in the guinea pig APC were studied by direct visualization of axons stained and quantitatively analyzed by intracellular biocytin injection in vivo. Results: The observations illustrated that axon collaterals of the individual cells were widely and spatially distrib- uted within the PC, and sometimes also showed a long associational projection to the olfactory bulb (OB). The data showed that long associational axons were both rostrally and caudally directed throughout the PC, and the intrinsic associational fibers of pyramidal cells in the APC are omnidirectional connections in the PC. Within the PC, associa- tional axons typically followed rather linear trajectories and irregular bouton distributions. Quantitative data of the axon collaterals of two pyramidal cells in the APC showed that the average length of axonal collaterals was 101 mm, out of which 79 mm (78% of total length) were distributed in the PC. -

The Connexions of the Amygdala
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.2.137 on 1 April 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 137 The connexions of the amygdala W. M. COWAN, G. RAISMAN, AND T. P. S. POWELL From the Department of Human Anatomy, University of Oxford The amygdaloid nuclei have been the subject of con- to what is known of the efferent connexions of the siderable interest in recent years and have been amygdala. studied with a variety of experimental techniques (cf. Gloor, 1960). From the anatomical point of view MATERIAL AND METHODS attention has been paid mainly to the efferent connexions of these nuclei (Adey and Meyer, 1952; The brains of 26 rats in which a variety of stereotactic or Lammers and Lohman, 1957; Hall, 1960; Nauta, surgical lesions had been placed in the diencephalon and and it is now that there basal forebrain areas were used in this study. Following 1961), generally accepted survival periods of five to seven days the animals were are two main efferent pathways from the amygdala, perfused with 10 % formol-saline and after further the well-known stria terminalis and a more diffuse fixation the brains were either embedded in paraffin wax ventral pathway, a component of the longitudinal or sectioned on a freezing microtome. All the brains were association bundle of the amygdala. It has not cut in the coronal plane, and from each a regularly spaced generally been recognized, however, that in studying series was stained, the paraffin sections according to the Protected by copyright. the efferent connexions of the amygdala it is essential original Nauta and Gygax (1951) technique and the frozen first to exclude a contribution to these pathways sections with the conventional Nauta (1957) method. -
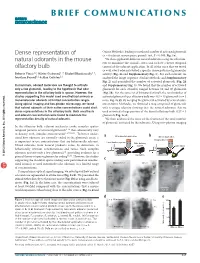
Dense Representation of Natural Odorants in the Mouse Olfactory Bulb
BRIEF COMMUNICATIONS Dense representation of Online Methods), leading to a reduced number of activated glomeruli (n = 6 odorant-mouse pairs, paired t test, P < 0.005; Fig. 1e). natural odorants in the mouse We then applied 40 different natural odorants using the olfactom- eter to minimize the animal’s stress and to have a better temporal olfactory bulb control of the odorant application. In all of the mice that we tested (n = 8), every odorant evoked a specific dense pattern of glomerular Roberto Vincis1,2, Olivier Gschwend1–3, Khaleel Bhaukaurally1–3, activity (Fig. 2a and Supplementary Fig. 1). For each odorant, we Jonathan Beroud1,2 & Alan Carleton1,2 analyzed the image sequence (Online Methods and Supplementary Fig. 2) and quantified the number of activated glomeruli (Fig. 2b In mammals, odorant molecules are thought to activate and Supplementary Fig. 3). We found that the number of activated only a few glomeruli, leading to the hypothesis that odor glomeruli for each stimulus ranged between 10 and 40 glomeruli representation in the olfactory bulb is sparse. However, the (Fig. 2b). For the same set of 30 natural stimuli, the total number of studies supporting this model used anesthetized animals or activated glomeruli per olfactory bulb was 443 ± 15 glomeruli (n = 5 monomolecular odorants at limited concentration ranges. mice; Fig. 2c,d). By merging the glomeruli activated by several odor- Using optical imaging and two-photon microscopy, we found ants (Online Methods), we obtained a map composed of glomeruli that natural odorants at their native concentrations could elicit with a unique identity showing that the natural odorants that we dense representations in the olfactory bulb. -

Functional Organization of Sensory Input to the Olfactory Bulb Glomerulus Analyzed by Two-Photon Calcium Imaging
Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging Matt Wachowiak*, Winfried Denk†, and Rainer W. Friedrich†‡ *Department of Biology, Boston University, 5 Cummington Street, Boston, MA 02215; and †Department of Biomedical Optics, Max Planck Institute for Medical Research, Jahnstrasse 29, D-69120 Heidelberg, Germany Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved May 10, 2004 (received for review January 20, 2004) Glomeruli in the olfactory bulb are anatomically discrete modules OSNs distinguished by histochemical markers terminate in dis- receiving input from idiotypic olfactory sensory neurons. To ex- crete subregions of the axonal compartment (36, 37). These data amine the functional organization of sensory inputs to individual raise the possibility that functionally distinct subdivisions exist .glomeruli, we loaded olfactory sensory neurons with a Ca2؉ within a glomerulus indicator and measured odorant-evoked presynaptic Ca2؉ signals Direct measurements of the activity of glomerular afferents within single glomeruli by using two-photon microscopy in anaes- have been difficult because most functional measures of intra- thetized mice. Odorants evoked patterns of discrete Ca2؉ signals glomerular activity lack sufficient spatial resolution or cellular throughout the neuropil of a glomerulus. Across glomeruli, Ca2؉ specificity. Here, we loaded OSNs with a fluorescent Ca2ϩ signals occurred with equal probability in all glomerular regions. indicator and measured patterns of afferent presynaptic activity ؉ Within single glomeruli, the pattern of intraglomerular Ca2 sig- within glomeruli by two-photon Ca2ϩ imaging in anaesthetized nals was indistinguishable for stimuli of different duration, iden- mice. Our data indicate that OSN input to individual glomeruli tity, and concentration. -

Olfaction and Olfactory Learning in Drosophila: Recent Progress Andre´ Fiala
Olfaction and olfactory learning in Drosophila: recent progress Andre´ Fiala The olfactory system of Drosophila resembles that of experience. For example, an odor repetitively paired with vertebrates in its overall anatomical organization, but is a food reward becomes attractive. Conversely, an odor considerably reduced in terms of cell number, making it an ideal that often occurs concurrently with a punishment model system to investigate odor processing in a brain becomes a predictor for a negative situation and will be [Vosshall LB, Stocker RF: Molecular architecture of smell avoided. Drosophila melanogaster can easily perform such and taste in Drosophila. Annu Rev Neurosci 2007, 30:505- learning tasks and represents an excellent organism to 533]. Recent studies have greatly increased our knowledge investigate the neuronal mechanisms underlying such about odor representation at different levels of integration, from olfactory learning processes for two reasons. First, con- olfactory receptors to ‘higher brain centers’. In addition, siderable progress has already been made during recent Drosophila represents a favourite model system to study the years in analyzing how odors are represented in the fly’s neuronal basis of olfactory learning and memory, and brain [1]. Second, the powerful genetic techniques by considerable progress during the last years has been made in which structure and function of identified neurons can be localizing the structures mediating olfactory learning and observed and manipulated makes Drosophila an ideal memory [Davis RL: Olfactory memory formation in neurobiological model system to characterize a neuronal Drosophila: from molecular to systems neuroscience. Annu network that mediates olfactory learning and memory [2– Rev Neurosci 2005, 28:275-302; Gerber B, Tanimoto H, 4]. -

Medial Temporal Lobe (The Limbic System)
MEDIAL TEMPORAL LOBE (THE LIMBIC SYSTEM) On the medial surface of the temporal lobe are three structures critical for normal human functioning. From rostral to caudal, they are the olfactory cortex, the amygdala, and the hippocampus. We will look at the anatomy and function of each separately, although they are often grouped together as "the limbic system". A. The olfactory system: The olfactory system actually begins in the roof of the nasal cavity. The olfactory receptors are ciliated epithelial cells with an array of receptors capable of detecting thousands of different odors. However, just as with any sensory system, the receptor neurons themselves do not project to the cerebral hemispheres. Their axons project up through the cribiform plate of the skull to synapse on the dendrites of the mitral cells of the olfactory bulb. The axons of the olfactory receptors make up the elusive cranial nerve I. This fragile tract is susceptible to shearing forces in head trauma, and loss of smell is a surprisingly debilitating injury. Here is an example of a section through olfactory bulb. The olfactory bulb is not a simple relay (something which passively transmits the signal), but is a sophisticated structure in itself. The mitral cell- olfactory neuron synapse is actually within a tangle of axons and dendrites that is called a glomerulus. There is a second cell type tucked around these glomeruli which probably affects how the signal is transmitted. These cells are small and densely packed, which gives them the name "granule cells". However, they bear no relation to the granule cells of the cerebellum or cerebral cortex. -

Olfactory Maps, Circuits and Computations
Available online at www.sciencedirect.com ScienceDirect Olfactory maps, circuits and computations Andrew J Giessel and Sandeep Robert Datta Sensory information in the visual, auditory and somatosensory between local positional features to extract information systems is organized topographically, with key sensory features like object identity, depth and motion [4–6]. Unlike the ordered in space across neural sheets. Despite the existence of a small number of continuous sensory parameters that spatially stereotyped map of odor identity within the olfactory characterize vision, audition and touch (such as position, bulb, it is unclear whether the higher olfactory cortex uses frequency and amplitude), olfactory parameter space is topography to organize information about smells. Here, we poorly defined and highly multidimensional [7]. For review recent work on the anatomy, microcircuitry and example, any given monomolecular odorant can be neuromodulation of two higher-order olfactory areas: the described in terms of its functional groups, molecular piriform cortex and the olfactory tubercle. The piriform is an weight, chain length, bond substitution, resonance fre- archicortical region with an extensive local associational network quency or any number of additional chemical descrip- that constructs representations of odor identity. The olfactory tors. Furthermore, olfactory space is inherently tubercle is an extension of the ventral striatum that may use discrete — not only are individual odorants structurally reward-based learning rules to encode odor valence. We argue unique but many of the molecular descriptors typically that in contrast to brain circuits for other sensory modalities, both used for individual odorants (such as functional group or the piriform and the olfactory tubercle largely discard any bond substitution) cannot be mapped continuously in topography present in the bulb and instead use distributive any scheme for chemical space. -

Rhesus Monkey Brain Atlas Subcortical Gray Structures
Rhesus Monkey Brain Atlas: Subcortical Gray Structures Manual Tracing for Hippocampus, Amygdala, Caudate, and Putamen Overview of Tracing Guidelines A) Tracing is done in a combination of the three orthogonal planes, as specified in the detailed methods that follow. B) Each region of interest was originally defined in the right hemisphere. The labels were then reflected onto the left hemisphere and all borders checked and adjusted manually when necessary. C) For the initial parcellation, the user used the “paint over function” of IRIS/SNAP on the T1 template of the atlas. I. Hippocampus Major Boundaries Superior boundary is the lateral ventricle/temporal horn in the majority of slices. At its most lateral extent (subiculum) the superior boundary is white matter. The inferior boundary is white matter. The anterior boundary is the lateral ventricle/temporal horn and the amygdala; the posterior boundary is lateral ventricle or white matter. The medial boundary is CSF at the center of the brain in all but the most posterior slices (where the medial boundary is white matter). The lateral boundary is white matter. The hippocampal trace includes dentate gyrus, the CA3 through CA1 regions of the hippocamopus, subiculum, parasubiculum, and presubiculum. Tracing A) Tracing is done primarily in the sagittal plane, working lateral to medial a. Locate the most lateral extent of the subiculum, which is bounded on all sides by white matter, and trace. b. As you page medially, tracing the hippocampus in each slice, the superior, anterior, and posterior boundaries of the hippocampus become the lateral ventricle/temporal horn. c. Even further medially, the anterior boundary becomes amygdala and the posterior boundary white matter. -

Variants of Olfactory Memory and Their Dependencies on the Hippocampal Formation
The Journal of Neuroscience, February 1995, f5(2): 1162-i 171 Variants of Olfactory Memory and Their Dependencies on the Hippocampal Formation Ursula Sttiubli,’ To-Tam Le,2 and Gary Lynch* ‘Center for Neural Science, New York University, New York, New York 10003 and *Center for the Neurobiology of Learning and Memory, University of California, Irvine, California 92717 Olfactory memory in control rats and in animals with entor- pal pyramidal cells (e.g., Hjorth-Simonsen, 1972; Witter, 1993). hinal cortex lesions was tested in four paradigms: (1) a known Moreover, physiological activity in rat hippocampus becomes correct odor was present in a group of familiar but nonre- synchronized with that in the olfactory bulb and cortex during warded odors, (2) six known correct odors were simulta- odor sampling(Macrides et al., 1982). Theseobservations have neously present in a maze, (3) correct responses required prompted speculationand experimentation concerning the pos- the learning of associations between odors and objects, and sible contributions of the several stagesof the olfactory-hip- (4) six odors, each associated with a choice between two pocampal circuit to the encoding and use of memory. Lesions objects, were presented simultaneously. Control rats had no to the lateral entorhinal cortex, which separatethe hippocampus difficulty with the first problem and avoided repeating se- from its primary source of olfactory input, did not detectably lections in the second; this latter behavior resembles that affect the ability of rats to perform odor discriminations learned reported for spatial mazes but, in the present experiments, prior to surgery although they did disrupt the learning of new was not dependent upon memory for the configuration of discriminations (Staubli et al., 1984, 1986).This result suggested pertinent cues. -

Odour Discrimination Learning in the Indian Greater Short-Nosed Fruit Bat
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb175364. doi:10.1242/jeb.175364 RESEARCH ARTICLE Odour discrimination learning in the Indian greater short-nosed fruit bat (Cynopterus sphinx): differential expression of Egr-1, C-fos and PP-1 in the olfactory bulb, amygdala and hippocampus Murugan Mukilan1, Wieslaw Bogdanowicz2, Ganapathy Marimuthu3 and Koilmani Emmanuvel Rajan1,* ABSTRACT transferred directly from the olfactory bulb to the amygdala and Activity-dependent expression of immediate-early genes (IEGs) is then to the hippocampus (Wilson et al., 2004; Mouly and induced by exposure to odour. The present study was designed to Sullivan, 2010). Depending on the context, the learning investigate whether there is differential expression of IEGs (Egr-1, experience triggers neurotransmitter release (Lovinger, 2010) and C-fos) in the brain region mediating olfactory memory in the Indian activates a signalling cascade through protein kinase A (PKA), greater short-nosed fruit bat, Cynopterus sphinx. We assumed extracellular signal-regulated kinase-1/2 (ERK-1/2) (English and that differential expression of IEGs in different brain regions may Sweatt, 1997; Yoon and Seger, 2006; García-Pardo et al., 2016) and orchestrate a preference odour (PO) and aversive odour (AO) cyclic AMP-responsive element binding protein-1 (CREB-1), memory in C. sphinx. We used preferred (0.8% w/w cinnamon which is phosphorylated by ERK-1/2 (Peng et al., 2010). powder) and aversive (0.4% w/v citral) odour substances, with freshly Activated CREB-1 induces expression of immediate-early genes prepared chopped apple, to assess the behavioural response and (IEGs), such as early growth response gene-1 (Egr-1) (Cheval et al., induction of IEGs in the olfactory bulb, hippocampus and amygdala.