For Review Only Journal of Zoological Systematics and Evolutionary Research Page 2 of 47
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Novel Rhabdovirus Infecting Newly Discovered Nycteribiid Bat Flies
www.nature.com/scientificreports OPEN Kanyawara Virus: A Novel Rhabdovirus Infecting Newly Discovered Nycteribiid Bat Flies Received: 19 April 2017 Accepted: 25 May 2017 Infesting Previously Unknown Published: xx xx xxxx Pteropodid Bats in Uganda Tony L. Goldberg 1,2,3, Andrew J. Bennett1, Robert Kityo3, Jens H. Kuhn4 & Colin A. Chapman3,5 Bats are natural reservoir hosts of highly virulent pathogens such as Marburg virus, Nipah virus, and SARS coronavirus. However, little is known about the role of bat ectoparasites in transmitting and maintaining such viruses. The intricate relationship between bats and their ectoparasites suggests that ectoparasites might serve as viral vectors, but evidence to date is scant. Bat flies, in particular, are highly specialized obligate hematophagous ectoparasites that incidentally bite humans. Using next- generation sequencing, we discovered a novel ledantevirus (mononegaviral family Rhabdoviridae, genus Ledantevirus) in nycteribiid bat flies infesting pteropodid bats in western Uganda. Mitochondrial DNA analyses revealed that both the bat flies and their bat hosts belong to putative new species. The coding-complete genome of the new virus, named Kanyawara virus (KYAV), is only distantly related to that of its closest known relative, Mount Elgon bat virus, and was found at high titers in bat flies but not in blood or on mucosal surfaces of host bats. Viral genome analysis indicates unusually low CpG dinucleotide depletion in KYAV compared to other ledanteviruses and rhabdovirus groups, with KYAV displaying values similar to rhabdoviruses of arthropods. Our findings highlight the possibility of a yet- to-be-discovered diversity of potentially pathogenic viruses in bat ectoparasites. Bats (order Chiroptera) represent the second largest order of mammals after rodents (order Rodentia). -

Bat Rabies Surveillance in Europe J
Zoonoses and Public Health SPECIALISSUE–BATS Bat Rabies Surveillance in Europe J. Schatz1, A. R. Fooks2, L. McElhinney2, D. Horton2, J. Echevarria3,S.Va´ zquez-Moron3, E. A. Kooi4, T. B. Rasmussen5,T.Mu¨ ller1 and C. M. Freuling1 1 Institute of Molecular Biology, WHO Collaborating Centre for Rabies Surveillance and Research, Friedrich-Loeffler-Institute, Federal Research Institute for Animal Health, Greifswald-Insel Riems, Germany 2 Wildlife Zoonoses and Vector Borne Diseases Research Group, Animal Health and Veterinary Laboratories Agency (Weybridge), Surrey, UK 3 Instituto de Salud Carlos III, Majadahonda, Madrid, Spain 4 Central Veterinary Institute of Wageningen UR, Lelystad, The Netherlands 5 DTU National Veterinary Institute, Lindholm, Kalvehave, Denmark Impacts • Rabies in European bats is caused by at least four different lyssavirus species that seem to have an association with certain bat species, that is, the Serotine bat (EBLV-1), the Daubenton’s and Pond bat (EBLV-2), Schreiber’s long-fingered bat (WCBV) and the Natterer’s bat (BBLV). • Almost all bat rabies cases were detected in dead or clinically affected bats i.e. passive surveillance. This is a much more sensitive approach than active surveillance where oral swab samples taken from individuals randomly captured during planned surveys were screened for viral RNA and/or virus. • The focus of bat rabies surveillance should be on testing of dead or moribund animals, and resulting positive FAT results should be further analysed to identify the lyssavirus species and all bats submitted for testing should be identified to species level. Keywords: Summary Rabies; bats; lyssavirus; surveillance Rabies is the oldest known zoonotic disease and was also the first recognized bat Correspondence: associated infection in humans. -

(Townsend's Big-Eared Bat), Eptesicus Fuscus
América do Norte Antrozous pallidus (Pallid), Corynorhinus townsendii (Townsend's big-eared bat), Eptesicus fuscus (Big brown), Euderma maculatum (Spotted), Eumops floridanus (Bonneted), Eumops perotis (Western mastiff), Eumops underwoodi (Underwood's bonneted), Lasionycteris noctivagans (Silver- haired), Lasiurus blossevilli (Western red), Lasiurus borealis (Eastern red), Lasiurus cinereus (Hoary), Lasiurus ega (Southern yellow), Lasiurus intermedius (Northern yellow), Lasiurus seminolus (S eminole bat), Lasiurus xanthinus (Western yellow), Macrotus californicus (California leaf-nosed), Molossus molossus (Pallas's mastiff), Mormoops megalophylla (Ghost faced), Myotis austroriparius (Southeastern myotis), Myotis californicus (California myotis), Myotis ciliolabrum (Western Small-footed), Myotis evotis (Long- eared myotis), Myotis grisescens (Gray), Myotis leibii (Small-footed), Myotis lucifugus (Little brown), Myotis occultus (Arizona myotis), Myotis septentrionalis (Northern long-eared myotis), Myotis sodalis (Indiana), Myotis thysanodes (Fringed myotis), Myotis velifer (Cave myotis), Myotis volans (Long-legged myotis), Myotis yumanensis (Yuma myotis), Nycticeius humeralis (Evening), Nyctinomops femorosaccus (Pocketed free-tailed), Nyctinomops macrotis (Big free-tailed), Parastrellus hesperus (Western pipistrelle), Perimyotis subflavus (Tricolored), Tadarida brasiliensis (Mexican free-tailed) Reino unido e Europa Barbastella barbastellus (Western barbastelle)*, Eptesicus isabellinus (Meridional serotine), Eptesicus isabellinus (Meridional -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Espécies Potencialmente Ameaçadas 2019 VF4 Pivott.Xlsx
List of Potentially Impacted Species by EDP activities Legenda (1) threat levels: i. Critically endangered (CR); ii. Endangered (EN); iii. Vulnerable (VU); iv. Near threatened (NT) and v. Least concern (LC) 31/12/2019 Species IUCN Red List of Threatened Species (1) National Red List Occurrence Scientific name Common name (EN) Common name (country of occurrence) Reino/Kingdom Classe/Class 2020‐01 latest assessment National Red List Country Tecnology Acanthochelys spixii Black Spiny‐necked Swamp Turtle cágado‐feio Animalia Reptilia NT 1‐ago‐06 Brazil Windpower Acanthodactylus erythrurus Animalia Reptilia LC 14‐dez‐08 Portugal Windpower Spiny‐footed Lizard Lagartixa‐de‐dedos‐denteados NT Accipiter gentilis Northern Goshawk Açor Animalia Aves LC 31‐mar‐15 Portugal Windpower VU Accipiter gentilis Northern Goshawk Azor común Animalia Aves LC 31‐mar‐15 NE Spain Windpower Accipiter gentilis Animalia Aves LC 31‐mar‐15 n/a Poland Windpower Northern Goshawk Jastrząb zwyczajny Accipiter gentilis Northern Goshawk Azor común Animalia Aves LC 31‐mar‐15 NE Spain Transmision grid‐ Renew Accipiter gentilis Northern Goshawk Azor Aves LC 31‐mar‐15 Spain Solar FV Animalia NT Accipiter nisus Eurasian sparrowhawk Gavião Animalia Aves LC 1‐out‐16 Portugal Windpower LC Accipiter nisus Eurasian sparrowhawk Gavilán común Animalia Aves LC 1‐out‐16 NE Spain Windpower Accipiter nisus Eurasian sparrowhawk Eurasian sparrowhawk Animalia Aves LC 1‐out‐16 France Windpower LC Eurasian Sparrowhawk Eurasian Sparrowhawk Accipiter nisus Animalia Aves LC 1‐out‐16 Italy Windpower -

African Bat Conservation News
Volume 36 African Bat Conservation News November 2014 ISSN 1812-1268 © ECJ Seamark, 2014 (AfricanBats) Above: A female Geoffroy’s Horseshoe Bat (Rhinolophus clivosus) caught in the Telperion Nature Reserve, Mpumalanga, South Africa on the 21 February 2014, with collectors number ECJS-42/2014. Inside this issue: Scientific contributions The first record of the African Sheath-tailed Bat Coleura afra (Peters, 1852) (Mammalia, Chiroptera) in 2 Cameroon with information on its ecology Recent Literature Papers 4 Notice Board Conferences 15 Call for contributions 15 Download and subscribe to African Bat Conservation News published by AfricanBats at: www.africanbats.org The views and opinions expressed in articles are no necessarily those of the editor or publisher. Articles and news items appearing in African Bat Conservation News may be reprinted, provided the author’s and newsletter refer- ence are given. African Bat Conservation News November 2014 vol. 36 2 ISSN 1812-1268 Scientific contributions THE FIRST RECORD OF THE AFRICAN SHEatH-taILED Bat Coleura afra (PETERS, 1852) (MAMMALIA, CHIROPTERA) IN CAMEROON WITH INFORmatION ON ITS ECOLOGY MATTHEW LEBRETON1,2, ERIC MOISE BAKWO FILS3*, JEAN MICHEL TAKUO2 and JOSEPH LE DOUX DIFFO JOSEPH DIFFO LEDOUX2 1 Mosaic, BP 35322, Yaoundé, CAMEROON. 2 Global Viral Cameroon, BP 7039, Yaoundé, CAMEROON. 3 Department of Biological Sciences; Faculty of Sciences; University of Maroua; CAMEROON *Corresponding author. E-mail address: [email protected] Coleura afra (Peters, 1852) is known from different localities across portions of sub-Saharan Africa. A colony of about 5,000 individuals of this species was recently identified in a cave, south east of Edea in the Littoral Region of Cameroon). -

Os Nomes Galegos Dos Morcegos 2018 2ª Ed
Os nomes galegos dos morcegos 2018 2ª ed. Citación recomendada / Recommended citation: A Chave (20182): Os nomes galegos dos morcegos. Xinzo de Limia (Ourense): A Chave. http://www.achave.ga"/wp#content/up"oads/achave_osnomes!a"egosdos$morcegos$2018.pd% Para a elaboración deste recurso léxico contouse coa colaboración de Lois de la Calle Carballal. Fotografía: orelludo cincento (Plecotus austriacus ). Autor: Jordi as. &sta o'ra est( su)eita a unha licenza Creative Commons de uso a'erto* con reco+ecemento da autor,a e sen o'ra derivada nin usos comerciais. -esumo da licenza: https://creativecommons.or!/"icences/'.#n #nd//.0/deed.!". Licenza comp"eta: https://creativecommons.or!/"icences/'.#n #nd//.0/"e!a"code0"an!ua!es. 1 !otas introdutorias " que contén este documento Na primeira edición deste documento (2016) fornecéronse denominacións galegas para as especies de morcegos : todas as que están presentes na Galiza (cando menos 2!) e máis de 500 especies do resto do mundo# Nesta segunda edición (2018) incorpórase o logo da %&a'e ao deseño do documento engádese algún nome galego máis e reescr*+ense as notas introdutorias# ,áis completa que a anterior nesta no'a edición ac&éganse nomes galegos para un total de 552 especies# A estrutura En primeiro lugar preséntase unha clasificación taxonómica que considera as superfamilias e familias de morcegos onde se apunta de maneira xeral os nomes dos morcegos que &ai en cada familia# / seguir 'én o corpo do documento unha listaxe onde se indica especie por especie, alén do nome cient*fico os nomes -

Bats Adapting to Manmade Structures
BATS ADAPTING TO HUMAN-MADE STRUCTURES - THE CASE OF THE “CONCESSÃO TRANSMONTANA” HIGHWAY BRIDGES - ID – 240 Susana Reis & Rui Rufino – Mãe d’água, Lda., R. Jorge Borges de Macedo 9A, Loja B, 2795-202 Linda- a-Velha, Portugal. [email protected] (Under a contract for ArqPais, Lda. and Autoestradas XXI) Resumo Os morcegos estão entre os grupos de espécies mais ameaçadas. Tradicionalmente, os morcegos abrigam-se em estruturas naturais como árvores velhas e grutas mas o uso de estruturas humanas tem aumentado. As pontes, em particular, podem ser utilizadas como abrigo pelos morcegos dependendo do tipo de estrutura e das características do habitat envolvente. Em alguns países a utilização deste tipo de estruturas é conhecida há mais de 30 anos. Em Portugal, foi apenas durante o primeiro ano do Programa de Monitorização da Auto- estrada da Concessão Transmontana que se detectou uma colónia de maternidade de grandes dimensões no viaduto sobre o Rio Tinhela. Este viaduto, que atravessa um dos maiores rios da região, é utilizado por várias centenas de morcegos-rabudos (Tadarida teniotis) que utilizam as fendas verticais das cornijas para se abrigarem pelo menos entre Julho e Novembro, não tendo sido observados morcegos durante o Inverno. No segundo ano de monitorização e durante todo o período de obras manteve-se a utilização do abrigo apesar da intensa perturbação resultante da presença humana, da circulação de máquinas e dos trabalhos de construção do novo viaduto estarem a decorrer a poucos metros do viaduto já existente. Durante os trabalhos de monitorização verificou-se que apenas uma das maiores pontes que atravessam os principais rios da região não é utilizada como abrigo por morcegos. -

African Bat Conservation News November 2009 ISSN 1812-1268
Volume 21 African Bat Conservation News November 2009 ISSN 1812-1268 Inside this issue: Notice board 1 Observations, Discussions 2 - 6 and Updates Flying foxes (Pteropodidae: 2 - 3 Pteropus) in the western Indian Ocean: a new regional initiative Scientific contributions 7 - 10 Bat Inventory of the Ivoloina 7 - 10 Foresty Station, Atsinana Region, Madagascar Recent Literature 11 - 17 © ECJ Seamark 2009 Above: Rendall’s Serotine bat (Neoromicia rendallii) (ECJS-44/2009) caught in the Chitabi area, Okavango Delta, Botswana. NOTICE BOARD Conferences 10th International Mammalogical Congress To be held at: Mendoza, Argentina, 9-14 August 2009 Further information: http://www.cricyt.edu.ar/imc10 nd 2 Berlin Bat Meeting: Bat Biology and Infectious Diseases To be held at: Berlin, Germany, 19– 21 February 2010. Further information: http://www.izw-berlin.de/ th 15 International Bat Research Conference To be held at: Prague, Czech Republic, 23– 27 August 2010. Further information: http://www.ibrc.cz Download and subscribe to African Bat The views and opinions expressed in articles are not necessarily those of the editor or Conservation News at: publisher. Articles and news items appearing in African Bat Conservation News may be reprinted, www.Africanbats.org provided the author’s and newsletter reference are given. Page 2 November 2009 vol. 21 African Bat Conservation News ISSN 1812-1268 OBSERVATIONS, DISCUSSIONS AND UPDATES FLYING FOXES (PTEROPODIDAE: PTEROPUS) IN THE WESTERN INDIAN OCEAN: A NEW REGIONAL INITIATIVE By: Richard K. B. Jenkins1,2 and Vikash Tatayah3 1 Madagasikara Voakajy, B.P. 5181, Antananarivo (101), Madagascar. 2 School of Biological Sciences, University of Aberdeen, Aberdeen, AB24 2TZ, United Kingdom. -
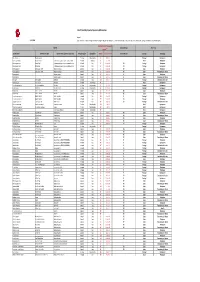
LEPI 2020 Pivot Table VF 20210406 (Version 1).Xlsb
List of Potentially Impacted Species by EDP activities Legend: 31/12/2020 (1) threat levels: i. Critically endangered (CR); ii. Endangered (EN); iii. Vulnerable (VU); iv. Near threatened (NT), Least concern (LC), Data deficient (DD), Not Applicable (NA) and Not Evaluated (NE) IUCN Red List of Threatened Species National Red List Occurrence Species (1) Scientific name Common name (EN) Common name (country of occurrence) Reino/Kingdom Classe/Class 2020‐01 latest assessment National Red List Country Tecnology Dianthus marizii Dianthus marizii ‐ Plantae Magnoliopsida LC 6‐abr‐11 Portugal Hydropower Coendou prehensilis Brazilian Porcupine ouriço‐cacheiro, porco‐espinho, cuandu e cuim Animalia Mammalia LC 10‐jun‐16 Brazil Windpower Hieraetus pennatus Booted Eagle A águia‐pequena, águia‐calçada ou águia‐de‐botas Animalia Aves LC 31‐mar‐15 NT Portugal Windpower Hieraetus pennatus Booted Eagle A águia‐pequena, águia‐calçada ou águia‐de‐botas Animalia Aves LC 31‐mar‐15 NT Portugal Hydropower Merops apiaster European Bee‐eater Abejaruco europeo Animalia Aves LC 31‐mar‐15 NE Spain Windpower Merops apiaster European Bee‐eater Abejaruco europeo Animalia Aves LC 31‐mar‐15 NE Spain Transmision grid‐ Renew Pernis apivorus Abejero europeo Animalia Aves LC 1‐out‐16 LC Spain Windpower Pernis apivorus Abejero europeo Animalia Aves LC 1‐out‐16 LC Spain Transmision grid‐ Renew Otis tarda Great bustard Abetarda Animalia Aves VU 1‐out‐17 EN Portugal Distribution Grid ‐activ Oxydoras niger ripsaw catfish Abotoado Animalia Actinopterygii NE Brazil Hydropower Santolina -

DPCC Species Dictionary V1.30
DPCC Species Dictionary v1.30 DPCC Species Dictionary v1.30 Status Host Common Name Host Species Order Family Genus Species ITIS Number Species Type Accepted shikra Accipiter badius Accipitriformes Accipitridae Accipiter badius 558390 Avian Accepted levant sparrowhawk Accipiter brevipes Accipitriformes Accipitridae Accipiter brevipes 175312 Avian Accepted chestnut-flanked sparrowhawk Accipiter castanilius Accipitriformes Accipitridae Accipiter castanilius 175314 Avian Accepted Cooper's hawk Accipiter cooperii Accipitriformes Accipitridae Accipiter cooperii 175309 Avian Accepted northern goshawk Accipiter gentilis Accipitriformes Accipitridae Accipiter gentilis 175300 Avian Accepted Japanese sparrowhawk Accipiter gularis Accipitriformes Accipitridae Accipiter gularis 175322 Avian Accepted Eurasian sparrowhawk Accipiter nisus Accipitriformes Accipitridae Accipiter nisus 175333 Avian Accepted sharp-shinned hawk Accipiter striatus Accipitriformes Accipitridae Accipiter striatus 175304 Avian Accepted African goshawk Accipiter tachiro Accipitriformes Accipitridae Accipiter tachiro 175344 Avian Accepted crested goshawk Accipiter trivirgatus Accipitriformes Accipitridae Accipiter trivirgatus 175347 Avian Accepted golden eagle Aquila chrysaetos Accipitriformes Accipitridae Aquila chrysaetos 175407 Avian Accepted eastern imperial eagle Aquila heliaca Accipitriformes Accipitridae Aquila heliaca 175413 Avian Accepted common buzzard Buteo buteo Accipitriformes Accipitridae Buteo buteo 175383 Avian Accepted broad-winged hawk Buteo platypterus Accipitriformes -

Kin Structure and Roost Fidelity in Greater Noctule Bats
bioRxiv preprint doi: https://doi.org/10.1101/675215; this version posted February 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Kin structure and roost fidelity in greater noctule bats João D. Santos1,2,*, Christoph F.J. Meyer1,3, Carlos Ibáñez4, Ana G. Popa-Lisseanu4 & Javier Juste4,5 1 Centre for Ecology, Evolution and Environmental Changes (cE3c), Faculty of Sciences, University of Lisbon, 1749-016 Lisbon, Portugal 2 UMR AGAP, CIRAD, F-34398 Montpellier, France 3 School of Science, Engineering and Environment, University of Salford, Salford, M5 4WT, United Kingdom 4 Department of Evolutionary Ecology, Estación Biológica de Doñana (CSIC), Avenida Américo Vespucio 26, 41092 Seville, Spain 5 Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública, CIBERESP, Spain *Correspondent: João Santos, present address: Cirad, AGAP, TA A-108 / 03, Avenue Agropolis, 34398 Montpellier Cedex 5, France; E-mail: [email protected], Tel: +33623720645 Running Header: Kin structure of the greater noctule bioRxiv preprint doi: https://doi.org/10.1101/675215; this version posted February 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Santos et al. Kin structure and roost fidelity in giant noctule bats 1 ABSTRACT 2 Roost fidelity is an important aspect of mammalian biology.