(CANSA) Fact Sheet on Cancer of Connective Tissue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soft Tissue Cytopathology: a Practical Approach Liron Pantanowitz, MD
4/1/2020 Soft Tissue Cytopathology: A Practical Approach Liron Pantanowitz, MD Department of Pathology University of Pittsburgh Medical Center [email protected] What does the clinician want to know? • Is the lesion of mesenchymal origin or not? • Is it begin or malignant? • If it is malignant: – Is it a small round cell tumor & if so what type? – Is this soft tissue neoplasm of low or high‐grade? Practical diagnostic categories used in soft tissue cytopathology 1 4/1/2020 Practical approach to interpret FNA of soft tissue lesions involves: 1. Predominant cell type present 2. Background pattern recognition Cell Type Stroma • Lipomatous • Myxoid • Spindle cells • Other • Giant cells • Round cells • Epithelioid • Pleomorphic Lipomatous Spindle cell Small round cell Fibrolipoma Leiomyosarcoma Ewing sarcoma Myxoid Epithelioid Pleomorphic Myxoid sarcoma Clear cell sarcoma Pleomorphic sarcoma 2 4/1/2020 CASE #1 • 45yr Man • Thigh mass (fatty) • CNB with TP (DQ stain) DQ Mag 20x ALT –Floret cells 3 4/1/2020 Adipocytic Lesions • Lipoma ‐ most common soft tissue neoplasm • Liposarcoma ‐ most common adult soft tissue sarcoma • Benign features: – Large, univacuolated adipocytes of uniform size – Small, bland nuclei without atypia • Malignant features: – Lipoblasts, pleomorphic giant cells or round cells – Vascular myxoid stroma • Pitfalls: Lipophages & pseudo‐lipoblasts • Fat easily destroyed (oil globules) & lost with preparation Lipoma & Variants . Angiolipoma (prominent vessels) . Myolipoma (smooth muscle) . Angiomyolipoma (vessels + smooth muscle) . Myelolipoma (hematopoietic elements) . Chondroid lipoma (chondromyxoid matrix) . Spindle cell lipoma (CD34+ spindle cells) . Pleomorphic lipoma . Intramuscular lipoma Lipoma 4 4/1/2020 Angiolipoma Myelolipoma Lipoblasts • Typically multivacuolated • Can be monovacuolated • Hyperchromatic nuclei • Irregular (scalloped) nuclei • Nucleoli not typically seen 5 4/1/2020 WD liposarcoma Layfield et al. -

The Health-Related Quality of Life of Sarcoma Patients and Survivors In
Cancers 2020, 12 S1 of S7 Supplementary Materials The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of A Nationwide Observational Study (PROSa) Martin Eichler, Leopold Hentschel, Stephan Richter, Peter Hohenberger, Bernd Kasper, Dimosthenis Andreou, Daniel Pink, Jens Jakob, Susanne Singer, Robert Grützmann, Stephen Fung, Eva Wardelmann, Karin Arndt, Vitali Heidt, Christine Hofbauer, Marius Fried, Verena I. Gaidzik, Karl Verpoort, Marit Ahrens, Jürgen Weitz, Klaus-Dieter Schaser, Martin Bornhäuser, Jochen Schmitt, Markus K. Schuler and the PROSa study group Includes Entities We included sarcomas according to the following WHO classification. - Fletcher CDM, World Health Organization, International Agency for Research on Cancer, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. 468 p. (World Health Organization classification of tumours). - Kurman RJ, International Agency for Research on Cancer, World Health Organization, editors. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. 307 p. (World Health Organization classification of tumours). - Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur Urol. 2016 Jul;70(1):106–19. - World Health Organization, Swerdlow SH, International Agency for Research on Cancer, editors. WHO classification of tumours of haematopoietic and lymphoid tissues: [... reflects the views of a working group that convened for an Editorial and Consensus Conference at the International Agency for Research on Cancer (IARC), Lyon, October 25 - 27, 2007]. 4. ed. -

Radiation-Associated Synovial Sarcoma
Radiation-Associated Synovial Sarcoma: Clinicopathologic and Molecular Analysis of Two Cases Jean-François Egger, M.D., Jean-Michel Coindre, M.D., Jean Benhattar, Ph.D., Philippe Coucke, M.D., Louis Guillou, M.D. University Institute of Pathology (J-FE, JB, LG) and Department of Radiooncology, University Hospital (PC), Lausanne, Switzerland; Bergonié Institute and University of Bordeaux II (J-MC), Bordeaux, France region, or viscera (1, 2). SS bears the t(X;18) (SYT- Development of a soft-tissue sarcoma is an infre- SSX) reciprocal translocation that seems to be spe- quent but well-known long-term complication of cific for this tumor type and can be routinely de- radiotherapy. Malignant fibrous histiocytomas, ex- tected in paraffin-embedded tissue using the traskeletal osteosarcomas, fibrosarcomas, malig- reverse transcriptase–polymerase chain reaction nant peripheral nerve sheath tumors, and angiosar- (RT-PCR; 3–6). Radiation-associated sarcomas are comas are most frequently encountered. Radiation- an infrequent but well-known long-term complica- associated synovial sarcomas are exceptional. We tion of radiotherapy (7–16). They occur in about report the clinicopathologic, immunohistochemi- 1/1000 patients who have undergone radiation cal, and molecular features of two radiation- therapy (7–11). Radiation-associated sarcomas are associated synovial sarcomas. One tumor developed defined as sarcomas arising in a previously irradi- in a 42-year-old female 17 years after external irra- ated field after a latency period of Ն2 years (12). diation was given for breast carcinoma; the other They usually show a more aggressive clinical course occurred in a 34-year-old female who was irradiated associated with shortened patient survival as com- at the age of 7 years for a nonneoplastic condition of pared with sporadic sarcomas (9–12, 14). -

Dermatofibrosarcoma Protuberans of the Parotid Gland -A Case Report
The Korean Journal of Pathology 2004; 38: 276-9 Dermatofibrosarcoma Protuberans of the Parotid Gland -A Case Report - Ok-Jun Lee∙David Y. Pi∙ Dermatofibrosarcoma protuberans (DFSP) typically presents during the early or mid-adult life, Daniel H. Jo∙Kyung-Ja Cho and the most common site of origin is the skin on the trunk and proximal extremities. DFSP of Sang Yoon Kim1∙Jae Y. Ro the parotid gland is extremely rare and only one case has been reported in the literature. We present here a case of a 30-year-old woman with DFSP occurring in the parotid gland, and we Departments of Pathology and discuss the differential diagnosis. The patient is alive and doing well one year after her operation. 1Otolaryngology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea Received : January 27, 2004 Accepted : July 5, 2004 Corresponding Author Jae Y. Ro, M.D. Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, 388-1 Pungnap-dong, Songpa-gu, Seoul 138-736, Korea Tel: 02-3010-4550 Fax: 02-472-7898 E-mail: [email protected] Key Words : Dermatofibrosarcoma Protuberans-Parotid Gland Epithelial tumors make up the majority of salivary gland neo- CASE REPORT plasms, while mesenchymal tumors of this organ are uncommon. Dermatofibrosarcoma protuberans (DFSP) of the salivary gland A 30-year-old woman came to the Otolaryngology Clinic at is exremely rare and only one case has been reported in the parotid the Asan Medical Center with a 2-year history of a slowly enlarg- gland.1 ing mass inferior to the left angle of the mandible. -

About Soft Tissue Sarcoma Overview and Types
cancer.org | 1.800.227.2345 About Soft Tissue Sarcoma Overview and Types If you've been diagnosed with soft tissue sarcoma or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is a Soft Tissue Sarcoma? Research and Statistics See the latest estimates for new cases of soft tissue sarcoma and deaths in the US and what research is currently being done. ● Key Statistics for Soft Tissue Sarcomas ● What's New in Soft Tissue Sarcoma Research? What Is a Soft Tissue Sarcoma? Cancer starts when cells start to grow out of control. Cells in nearly any part of the body can become cancer and can spread to other areas. To learn more about how cancers start and spread, see What Is Cancer?1 There are many types of soft tissue tumors, and not all of them are cancerous. Many benign tumors are found in soft tissues. The word benign means they're not cancer. These tumors can't spread to other parts of the body. Some soft tissue tumors behave 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 in ways between a cancer and a non-cancer. These are called intermediate soft tissue tumors. When the word sarcoma is part of the name of a disease, it means the tumor is malignant (cancer).A sarcoma is a type of cancer that starts in tissues like bone or muscle. Bone and soft tissue sarcomas are the main types of sarcoma. Soft tissue sarcomas can develop in soft tissues like fat, muscle, nerves, fibrous tissues, blood vessels, or deep skin tissues. -

Aneurysmal Fibrous Histiocytoma: a Case Report and Review of the Literature Devin M
Aneurysmal Fibrous Histiocytoma: A Case Report and Review of the Literature Devin M. Burr, DO,* Warren A. Peterson, DO,** Michael W. Peterson, DO*** *Dermatology Resident, 1st year, Aspen Dermatology Residency Program, Springville, UT **Program Director, Aspen Dermatology Residency Program, Springville, UT ***Dermatopathologist, Springville Dermatology, Springville, UT Disclosures: None Correspondence: Devin M. Burr, DO; [email protected] Abstract Dermatofibroma is one of the most common subcutaneous dermatologic tumors. In its classic variant, a dermatofibroma is easily recognized by dermatologists; however, studies have identified numerous variants of the dermatofibroma that do not present with a classic clinical picture. Aneurysmal fibrous histiocytoma, one of these variants, is not easily recognized given its bizarre growth and potentially malignant appearance. Microscopically, aneurysmal fibrous histiocytoma can be difficult to identify, as the lesion will display some similarities to a classic dermatofibroma along with distinguishing characteristics, like large blood-filled cavernous spaces. Aneurysmal fibrous histiocytoma is a benign lesion with a low risk for recurrence if adequately excised. In this paper, we present a case of aneurysmal fibrous histiocytoma and review the literature on this rare dermatofibroma variant and what to consider on the differential diagnosis. Introduction right scapula. He reported that it had been present subcutis (Figure 3). Immunohistochemical stains Dermatofibroma, also known as fibrous for about one year and initially appeared as a 1 mm FXIIIa, CD10, and CD68 confirmed that the histiocytoma, is a common dermatologic to 2 mm purple papule. It slowly grew for the first lesion was a histiocytic tumor (Figure 4). CD34 subcutaneous tumor. It represents roughly 3% six months and then rapidly enlarged in size over highlighted the vascular component. -

Soft Tissue Sarcoma: an Insight on Biomarkers at Molecular, Metabolic and Cellular Level
cancers Review Soft Tissue Sarcoma: An Insight on Biomarkers at Molecular, Metabolic and Cellular Level Serena Pillozzi 1,* , Andrea Bernini 2, Ilaria Palchetti 3 , Olivia Crociani 4, Lorenzo Antonuzzo 1,4, Domenico Campanacci 5 and Guido Scoccianti 6 1 Medical Oncology Unit, Careggi University Hospital, Largo Brambilla 3, 50134 Florence, Italy; lorenzo.antonuzzo@unifi.it 2 Department of Biotechnology, Chemistry and Pharmacy, University of Siena, Via Aldo Moro 2, 53100 Siena, Italy; [email protected] 3 Department of Chemistry Ugo Schiff, University of Florence, Via della Lastruccia 3, 50019 Sesto Fiorentino, Italy; ilaria.palchetti@unifi.it 4 Department of Experimental and Clinical Medicine, University of Florence, Largo Brambilla 3, 50134 Florence, Italy; olivia.crociani@unifi.it 5 Department of Health Science, University of Florence, Largo Brambilla 3, 50134 Florence, Italy; domenicoandrea.campanacci@unifi.it 6 Department of Orthopaedic Oncology and Reconstructive Surgery, University of Florence, Careggi University Hospital, Largo Brambilla 3, 50134 Florence, Italy; [email protected] * Correspondence: serena.pillozzi@unifi.it Simple Summary: Soft tissue sarcoma is a rare mesenchymal malignancy. Despite the advancements in the fields of radiology, pathology and surgery, these tumors often recur locally and/or with metastatic disease. STS is considered to be a diagnostic challenge due to the large variety of histologi- Citation: Pillozzi, S.; Bernini, A.; cal subtypes with clinical and histopathological characteristics which are not always distinct. One of Palchetti, I.; Crociani, O.; Antonuzzo, the important clinical problems is a lack of useful biomarkers. Therefore, the discovery of biomarkers L.; Campanacci, D.; Scoccianti, G. Soft that can be used to detect tumors or predict tumor response to chemotherapy or radiotherapy could Tissue Sarcoma: An Insight on help clinicians provide more effective clinical management. -

Dermatofibrosarcoma Protuberans
Oxford University Hospitals NHS Trust Dermatofibrosarcoma Protuberans (DFSP) Information for patients What is dermatofibrosarcoma protuberans (DFSP)? Dermatofibrosarcoma protuberans is a rare malignant (cancerous) tumour which can develop in the deep layers of the skin. It is classed as a soft tissue sarcoma. Dermatofibrosarcoma protuberans can be locally aggressive, which means it can affect the surrounding tissues nearby. There is no definite known cause for this type of tumour. Dermatofibrosarcoma protuberans can affect any age group but is most common between the ages of 20 to 50. It affects slightly more women than men. Diagnosis We will confirm your diagnosis once we have carried out ultrasound and MRI scans (imaging) and we have the histology (results) from the biopsy taken from the tumour, if this is needed. The dermatofibrosarcoma protuberans will also be ‘graded’, depending on how fast it is growing and how likely it is to spread to other parts of the body. As with all cancers, dermatofibrosarcoma protuberans can spread (metastasise) to other parts of the body. We will check for this very quickly after your diagnosis by taking CT or PET CT scans. Dermatofibrosarcoma protuberans can spread to the lungs so we will closely monitor your chest with X-rays at each clinic appointment during your follow-up. We may also take further CT scans if we feel these are needed. Treatment options The usual treatment for dermatofibrosarcoma protuberans is surgery to remove the tumour. Sometimes these tumours can have ‘tentacles’ which invade the areas surrounding the tumour, which means we may need to also remove a large area around the tumour. -

Rhabdomyosarcoma and Soft Tissue Sarcoma
A Handbook for Families Rhabdomyosarcoma and Soft Tissue Sarcoma ONCOLOGY SERIES A Handbook for Families Rhabdomyosarcoma and Soft Tissue Sarcoma ONCOLOGY SERIES Rhabdomyosarcoma and Soft Tissue Sarcoma A HANDBOOK FOR FAMILIES Author Kathleen Adlard, MN RN CPON® Barbara Humrick, MS RN CPNP CPON® Kelly Laschinger, RN CPNP Reviewers 2015–2016 Steering Council Parent Reviewer Lori Crossley Previous Contributors Margaret Bottcher, MN RN CPNP CPON® Violet Shen, MD David Tishler, MD Ann Watts, RN CNP ABOUT THIS COVER This cover is specially designed for your child to color and personalize. When your child finishes decorating the cover, return it to the clinic or doctor’s office where you received the handbook. Your child’s healthcare This handbook is published by the Association of Pediatric Hematology/Oncology Nurses (APHON) for educational purposes only. The material has been developed by sources believed to be reliable. The material is not intended to represent the only acceptable or safe treatment of rhabdomyosarcoma and soft tissue sarcoma. Under certain circumstances or conditions, additional or different treatment may be required. As new research and clinical experience expand the sources of information available concerning the treatment of rhabdomyosarcoma and soft tissue sarcoma, adjustments in treatment and drug therapy may be required. APHON makes no warranty, guarantee, or other representation, express or implied, concerning the validity or sufficiency of the treatments or related information contained in this handbook. APHON grants the purchaser of this handbook unrestricted permission to photocopy the handbook for educational use by the purchaser or the purchaser’s institution. Purchaser may not receive monetary gain from distributing photocopies of this product. -

Benign Extramedullary Myeloid Proliferations
Modern Pathology (2007) 20, 405–415 & 2007 USCAP, Inc All rights reserved 0893-3952/07 $30.00 www.modernpathology.org Benign extramedullary myeloid proliferations Dennis P O’Malley US Labs, Irvine, CA, USA Extramedullary proliferations of bone marrow elements are infrequently encountered in routine pathology practice. On occasion, they can present diagnostic difficulties when seen in unusual or unanticipated sites. This review serves to cover aspects of underlying embryogenesis of myeloid elements, as well as sites and circumstance of benign proliferations of myeloid elements along with their occasional confusion with neoplastic myeloid proliferations. Benign proliferations associated with hematologic disorders and hemato- poietic growth factors are discussed. Immunohistochemical evaluation of myeloid proliferations is considered as well. Modern Pathology (2007) 20, 405–415. doi:10.1038/modpathol.3800768; published online 2 March 2007 Keywords: extramedullary hematopoiesis; spleen; lymph node; myelolipoma; myeloid sarcoma; diagnosis The production of blood cells by the bone marrow is epididymis, ovaries and endometrium.1,2 It is these the basic function of the hematopoietic system. The circumstances and the proliferation of marrow appropriate proliferation and production of marrow elements in extramedullary sites that are the focus elements is fundamental to the control of numerous of this review. Evaluation of neoplastic myeloid body systems, because of the far-reaching effects of proliferations (NMP), in the context of differential the hematologic elements. Oxygen transport by red diagnosis, will also be briefly discussed. cells, coagulation by platelets and the nonspecific immune responses by granulocytes and monocytes are the essential functions, but there are numerous Benign other, more subtle functions of the hematopoietic system as well. -

Malignant Dermatofibroma
Modern Pathology (2013) 26, 256–267 256 & 2013 USCAP, Inc All rights reserved 0893-3952/13 $32.00 Malignant dermatofibroma: clinicopathological, immunohistochemical, and molecular analysis of seven cases Thomas Mentzel1, Thomas Wiesner2, Lorenzo Cerroni2, Markus Hantschke1, Heinz Kutzner1, Arno Ru¨ tten1, Michael Ha¨berle3, Michele Bisceglia4, Frederic Chibon5 and Jean-Michel Coindre5 1Dermatopathology Bodensee Friedrichshafen, Friedrichshafen, Germany; 2Deparment of Dermatology, Medical University of Graz, Graz, Austria; 3Dermatological Practice, Ku¨nzelsau, Germany; 4IRCCS-CROB, Referral Cancer Center of Basilicata, Rionero in Vulture (PZ), Italy and 5Bergonie Institute, InsermU916 and University Victor Segalen Bordeaux, Bordeaux, France Dermatofibroma (cutaneous fibrous histiocytoma) represents a common benign mesenchymal tumor, and numerous morphological variants have been described. Some variants of dermatofibroma are characterized by an increased risk of local recurrences, and there are a few reported metastasizing cases. Unfortunately, an aggressive behavior cannot be predicted reliably by morphology at the moment, and we evaluated the value of array-comparative genomic hybridization (CGH) in this setting. Seven cases of clinically aggressive dermatofibromas were identified, and pathological and molecular features were evaluated. The neoplasms occurred in four female and in three male patients (mean age was 33 years, range 2–65 years), and arose on the shoulder, buttock, temple, lateral neck, thigh, ankle, and cheek. The size of the neoplasms ranged from 1 to 9 cm (mean: 3 cm). An infiltration of the subcutis was seen in five cases. Two neoplasms were completely excised, whereas an incomplete or marginal excision was reported in the remaining cases. Local recurrences were seen in six cases (time to the first recurrence ranged from 8 months to 9 years). -
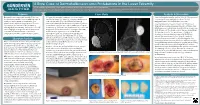
A Rare Case of Dermatofibrosarcoma Protuberans in the Lower
A Rare Case of Dermatofibrosarcoma Protuberans in the Lower Extremity Andrea J Cifaldi, DPM, Mitchell J Thompson, DPM, Matthew R Sieloff, DPM, Thomas S Roukis, DPM, PhD, FACFAS, Andrew D Elliott, DPM, JD (1) PGY-2, Podiatric Medicine and Surgery Residency Program, Gundersen Medical Foundation, La Crosse, WI; (2) PGY-3, Podiatric Medicine and Surgery Residency Program, Gundersen Medical Foundation, La Crosse, WI; (3) PGY-1, Podiatric Medicine and Surgery Residency Program, Gundersen Medical Foundation, La Crosse, WI; (4) Attending Staff, Gundersen Health System, La Crosse, WI Purpose Case Study Analysis & Discussion Dermatobrosarcoma protuberans (DFSP) is a rare, A 21-year-old otherwise healthy male presented with a Since rst being described by Taylor in 1890, DFSP has remained locally invasive soft tissue sarcoma with high rates of several year history of a darkened, enlarging soft tissue mass a b a rare malignancy accounting for less than 0.1% of all recurrence and metastatic potential when not on the medial ankle (Fig. 1). An MRI with and without malignancies and 1.8% of all soft tissue sarcomas.8, 9 identied early and treated appropriately. There are contrast was obtained that demonstrated a 2.4 x 3.7 x 4.0 cm The patient presented here was 21 years old at the time of few reports in the literature of this malignancy encapsulated supercial mass with a small region of capsule diagnosis and several years younger when the soft tissue mass aecting the lower extremity. The present case study disruption (Fig. 2). The mass was hyperintense on T2 and STIR initially appeared, making him younger than the peak age range aims to highlight strategies for clinical identication images and isointense to muscle on T1 images.