Efficacy of Ultrasound Imaging in the Evaluation of the Lisfranc Joint Complex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fractures Fractura Pathologica
Fractures Fractura pathologica Myeloma Fractura traumatica Fractura aperta/clausa Fractura simplex/multiplex Fractura comminutiva Fractura transversa/obliqua Fractura spiralis/longitudinalis Fractura compressiva/impressiva Fractura incuneata Infractio = f. partialis = f. incompleta Fractura cum dislocatione ad axim ad latus ad longitudinem cum contractione ad longitudinem cum distractione AO ClassificationAuthentic reports of fractures : 2 S 4220 Fractura colli chirurgici humeri l. dx. comminutiva AO 11-C3 Fracture Healing: 1: REPOSITIO = REDUCTIO fragmentorum CLOSED (short /long term) Fracture Healing: 2: FIXATIO = STABILISATIO fragmentorum PLASTER CAST INTERNAL FIXATION Fracture Healing: 2: FIXATIO = STABILISATIO fragmentorum INTERNAL FIXATION Fracture Healing: 2: FIXATIO = STABILISATIO fragmentorum Name the type of fracture A B C D E F Choose a bone and break it. Try to write as much detailed diagnosis as possible. Authentic reports :1 collement = severe damage of soft tissues Authentic reports :2 Fr. aperta TSCHERNE I - open fracture with small skin injury without its contusion - negligible bacterial contamination Profesor Dr. Harald Tscherne (1933), Traumatology Clinic, Hannover: Classification of fractures published in 1982, T. divides fracture into open and closed. The most important for him is the degree of the soft tissues damage. Authentic reports :3 1 A 45-year-old woman presented with a 3-month history of generalized body pains nonresponsive to analgesic agents. Along with low back pain, she had progressive difficulty in getting up from sitting and supine positions and in walking. There was no history of trauma or any medication intake. She is an orthodox believer who wears a black veil outdoors and is completely covered, with little exposure to the sun. An anteroposterior radio- graph of the pelvis showed an undisplaced transverse fracture of the shaft of both femurs. -

Biomechanical Comparison of Fixation Stability Using a Lisfranc Plate
Foot and Ankle Surgery 25 (2019) 71–78 Contents lists available at ScienceDirect Foot and Ankle Surgery journa l homepage: www.elsevier.com/locate/fas Biomechanical comparison of fixation stability using a Lisfranc plate $ versus transarticular screws a,b a,c, d d Nathan C. Ho , Sophia N. Sangiorgio *, Spenser Cassinelli , Stephen Shymon , d a,b a,c d John Fleming , Virat Agrawal , Edward Ebramzadeh , Thomas G. Harris a The J. Vernon Luck, Sr., M.D. Orthopaedic Research Center, Orthopaedic Institute for Children, in Alliance with UCLA, 403 W. Adams Blvd., Los Angeles, CA 90007, United States b University of Southern California Department of Biomedical Engineering, Los Angeles, CA, United States c University of California, Los Angeles Department of Orthopaedic Surgery, Los Angeles, CA, United States d Los Angeles County Harbor—UCLA Medical Center, Los Angeles, CA, United States A R T I C L E I N F O A B S T R A C T Article history: Background: To obtain adequate fixation in treating Lisfranc soft tissue injuries, the joint is commonly Received 3 March 2017 stabilized using multiple transarticular screws; however iatrogenic injury is a concern. Alternatively, two Received in revised form 28 July 2017 parallel, longitudinally placed plates, can be used to stabilize the 1st and 2nd tarsometatarsal joints; Accepted 8 August 2017 however this may not provide adequate stability along the Lisfranc ligament. Several biomechanical studies have comparedearliermethodsoffixation using platestothestandardtransarticularscrew fixationmethod, Keywords: highlighting the potential issue of transverse stability using plates. A novel dorsal plate is introduced, Lisfranc injury intended to provide transverse and longitudinal stability, without injury to the articular cartilage. -

An Old Mismanaged Lisfranc Injury Treated by Gradual Deformity Correction Followed by the Second-Stage Internal fixation
Strat Traum Limb Recon (2017) 12:59–62 DOI 10.1007/s11751-016-0273-3 CASE REPORT An old mismanaged Lisfranc injury treated by gradual deformity correction followed by the second-stage internal fixation 1 1 1 1 Mehraj D. Tantray • Khurshid Kangoo • Asif Nazir • Muzamil Baba • 1 1 1 Raja Rameez • Syed Tabish • Syed Shahnawaz Received: 29 May 2016 / Accepted: 27 December 2016 / Published online: 5 January 2017 Ó The Author(s) 2017. This article is published with open access at Springerlink.com Abstract The Lisfranc fracture-dislocation of the foot is Keywords Lisfranc injury Á Ilizarov Á Late diagnosis uncommon and diagnosis is often missed. The Lisfranc joint involves the articulation between medial cuneiform and base of the second metatarsal and is considered a Introduction keystone to structural integrity to the midfoot. The articu- lation has a stabilization effect on longitudinal and trans- Injuries to the tarsometatarsal joints are not common and verse arches of the foot. A neglected or untreated injury to represent less than 0.2% of all orthopaedic injuries with the Lisfranc joint can lead to secondary arthritis and sig- a reported incidence of 1 per 55,000 individuals [1]. The nificant morbidity and disability. We present a case of a injury is commonly missed due to gross swelling mask- neglected Lisfranc fracture-dislocation in a 28-year-old ing the deformity and subtle findings on radiological female patient who presented 3 months after injury. A evaluation which requires careful attention. Re-examina- staged treatment of distraction with an Ilizarov ring fixator tion after the decrease in oedema for persistent pain and followed in the second stage by the removal of ring fixator aggravation of pain or instability on stress examination and internal fixation with K wires was performed. -

Sport Following Lisfranc Injuries: a Systematic Review and Meta-Analysis
Foot and Ankle Surgery 25 (2019) 654–664 Contents lists available at ScienceDirect Foot and Ankle Surgery journal homepage: www.elsevier.com/locate/fas Return to sport following Lisfranc injuries: A systematic review and meta-analysis a, b c a Gregory Aidan James Robertson *, Kok Kiong Ang , Nicola Maffulli , Gary Keenan , d Alexander MacDonald Wood a Edinburgh Orthopaedic Trauma Unit, Royal Infirmary of Edinburgh, 51 Little France Crescent, Edinburgh EH16 4SA, United Kingdom b Edinburgh Medical School, University of Edinburgh, 47 Little France Crescent, Edinburgh EH16 4TJ, United Kingdom c Queen Mary University of London, Mile End Road, London E1 4NS, United Kingdom d Leeds General Infirmary, Great George St, Leeds LS1 3EX, United Kingdom A R T I C L E I N F O A B S T R A C T Article history: Background: Information regarding return rates (RR) and mean return times (RT) to sport following Received 25 April 2018 Lisfranc injuries remains limited. Received in revised form 17 July 2018 Methods: A systematic search of nine major databases was performed to identify all studies which Accepted 24 July 2018 recorded RR or RT to sport following lisfranc injuries. Results: Seventeen studies were included (n = 366). Keywords: For undisplaced (Stage 1) injuries managed nonoperatively (n = 35), RR was 100% and RT was 4.0 (0–15) Lisfranc wks. For stable minimally-displaced (Stage 2) injuries managed nonoperatively (n = 16), RR was 100% and Tarso-metarsal RT was 9.1 (4–14) wks. Mid-foot Return For the operatively-managed injuries, Percutaneous Reduction Internal Fixation (PRIF) (n = 42), showed Sport significantly better RR and RT compared to both: Open Reduction Internal Fixation (ORIF) (n = 139) (RR — Rate 98% vs 78%, p < 0.019; RT — 11.6 wks vs 19.6 wks, p < 0.001); and Primary Partial Arthrodesis (PPA) (n = 85) Time (RR — 98% vs 85%, p < 0.047; RT — 11.6 wks vs 22.0 wks, p < 0.002). -

Lisfranc Injury and Midfoot Arthritis
Dr Todd Gothelf www.orthosports.com.au 47‐49 Burwood Road, Concord 29‐31 Dora Street, Hurstville 119‐121 Lethbridge Street, Penrith 160 Belmore Road, Randwick Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Midfoot Sprains and Arthritis Todd K Gothelf Foot, Ankle, Shoulder Surgeon Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Midfoot Arthritis • Arthritis of Tarsometatarsal complex • Lisfranc Complex • Major Causes – Lisfranc sprain – Primary Arthritis – Inflammatory Disease Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Lisfranc Injury • One of the most common athletic foot injuries • Difficult Diagnosis • It will see you • Treatable When detected • Arthritis Preventable Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Lisfranc Joints • Tarsometatarsal joint complex • Increased awareness • Fairly common injury in sports • Up to 20% overlooked on initial radiographs • Left untreated, can lead to arthritis, prolonged pain, difficulty returning to sport Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Jacques Lisfranc • French Surgeon • Napolean Era • Described amputations through midfoot Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Mechanism of injury • Direct Injury‐ Crush to Midfoot • Indirect Injury‐ Twisting • With toes planted • Ankle in plantar flexion • Forceful abduction of the midfoot Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Anatomy • Medial cuneiform to the second metatarsal. • Intimately related to one another. • Bones offer primary stability • Held together by ligaments. Dr Todd Gothelf Shoulder, Foot & Ankle Surgery Anatomy‐ -

Tarsometatarsal Joint Injury/Arthritis John P
Tarsometatarsal joint injury/arthritis John P. Negrine FRACS Foot and ankle surgeon Dr John Negrine Adult Foot and Ankle surgeon Lisfranc injury • Named after a Napoleonic surgeon who didn’t actually describe the injury – he described am amputation through the joints Dr John Negrine Adult Foot and Ankle surgeon Jacques Lisfranc de St. Martin • 1790 – 1847 • “A surgeon and gynaecologist” • Trained as an assistant to Guillaume Dupuytren • “Pioneered operations including removal of the rectum, lithotomy in women as well as amputation of the cervix” Dr John Negrine Adult Foot and Ankle surgeon What do we call the “Lisfranc joints?” • The tarsometatarsal joints • Anatomy well known to you • The second metatarsal base is recessed and the strong ligament is on the plantar surface connecting the medial cuneiform to the base of the second metatarsal…the “Lisfranc ligament” Dr John Negrine Adult Foot and Ankle surgeon Lisfranc anatomy Dr John Negrine Adult Foot and Ankle surgeon Common injury • Commonly missed • Commonly underestimated • Long time to recovery • Don’t always do well! Dr John Negrine Adult Foot and Ankle surgeon Common ‐ ?Really? • No not really! • 0.2% of fractures • 1:70,000 people in a hospital catchment area • 20% are missed Dr John Negrine Adult Foot and Ankle surgeon Mechanism of injury • 43% Motor vehicle accidents • 24% from falls, jumps or twisting injury • 13% due to crush injury Dr John Negrine Adult Foot and Ankle surgeon Important to differentiate High velocity vs low velocity Dr John Negrine Adult Foot and Ankle surgeon -

Complex Cases
Repair and Fix the Lisfranc Joint Don’t Fuse It Gil R. Ortega, MD, MPH Sonoran Orthopaedic Trauma Surgeons Orthopaedic Trauma Director, Mayo Clinic Arizona Residency Program Vice Chair, Department of Surgery, Scottsdale Osborn Level 1 Trauma Center, Scottsdale, AZ, USA Disclosures • Founding Member, Orthopaedic Board of Advisors: Carbofix • Founding Member, Orthopaedic Board of Advisors: Artross Nanobone • Consultant: Smith and Nephew Why Fuse a joint that suffers a ligament injury without a fracture? • Do we fuse shoulder or elbow joints after dislocations without fractures? • Do we fuse hips after dislocations without fractures? • Do we fuse knees after knee dislocations with ligament injuries only? • Do we fuse ankles after ankle dislocations without fractures? • NO OF COURSE NOT…So why fuse the Lisfranc? Fusing a Lisfranc joint after a dislocation makes no physiologic sense • A Lisfranc injury is a ligamentous injury with or without associated fractures The Weak Argument to Fuse a Lisfranc is based on one study • Forty-one patients in prospective, randomized clinical trial comparing primary arthrodesis with ORIF • Patients followed for average of 42.5 months Evaluation was performed with clinical examination, radiography, American Orthopaedic Foot and Ankle Society (AOFAS) Midfoot Scale, a visual analog pain scale, and a clinical questionnaire The Weak Argument to Fuse a Lisfranc is based on one study • Twenty patients-ORIF and 21-Fusion • Two years postop, mean AOFAS Midfoot score was 68.6 points in ORIF and 88 points in the arthrodesis -

A Study of Functional Outcome of Lisfranc Fracture Dislocations
National Journal of Clinical Orthopaedics 2018; 2(4): 85-89 ISSN (P): 2521-3466 ISSN (E): 2521-3474 © Clinical Orthopaedics A study of functional outcome of lisfranc fracture www.orthoresearchjournal.com 2018; 2(4): 85-89 dislocations managed by various operative methods in Received: 12-08-2018 Accepted: 13-09-2018 rural south Indian population Dr. J Kumaran Postgraduate, Department of Dr. J Kumaran, Dr. R Neelakrishnan, Dr. V Bharathiselvan and Orthopaedics, Rajah Muthiah Dr. Anush Rao Medical College & Hospital, Annamalai University, Tamil Nadu, India Abstract This study is an attempt to analyse the midterm results of “functional outcome of lisfranc fracture Dr. R Neelakrishnan dislocations managed by various operative methods” in rural south Indian population. Lisfranc Fracture Professor & HOD, Department dislocations have low incidence as they are commonly missed. Painful malunion or impaired functions of Orthopaedics, Rajah Muthiah may result if not treated adequately. Anatomic reduction and internal fixation is recommended. The study Medical College & Hospital, involves 15 patients, aged 18 to 65 admitted to our hospital, Rajah Muthiah Medical College, Annamalai University, Chidambaram for period of 24 months from May 2016 to April 2018.Myerson’s Classification of lisfranc Tamil Nadu, India fracture disloaction was used to classify and manage the injuries accordingly. Patients were managed with surgical reduction (Open or closed) using Kwires/Screws. Post operatively immobilisation was done Dr. V Bharathiselvan using BK cast/slab for a period varying from 4 - 12 weeks and later Gradual weight bearing allowed after Lecturer, Department of 6 -8 weeks. AOFAS Midfoot score was used to document the prognosis. The study involves 15 patients, Orthopaedics, Rajah Muthiah Medical College & Hospital, 11 men and 4 women aged 18 to 65 years (mean = 31) admitted to our hospital, for period of 24 months Annamalai University, from May 2016 to April 2018 (mean follow up period - 9 months). -

Lisfranc Fracture Dislocation: a Review of a Commonly Missed Injury of the Midfoot Simon Lau,1 Michael Bozin,2 Tharsa Thillainadesan1
Review Lisfranc fracture dislocation: a review of a commonly missed injury of the midfoot Simon Lau,1 Michael Bozin,2 Tharsa Thillainadesan1 1Department of Orthopaedic ABSTRACT the cuboid. Stability at the Lisfranc joint is provided Surgery, Royal Melbourne Musculoskeletal trauma to the foot is a common by its osseous configuration. The middle cuneiform Hospital, Parkville, Victoria, Australia presentation to EDs. A Lisfranc fracture dislocation is recessed in comparison to the medial and lateral 2General Surgical and Trauma involves injury to the bony and soft tissue structures of cuneiforms, and this recess accommodates the base Registrar, Royal Melbourne the tarsometatarsal joint. While it is most commonly of the second metatarsal, creating a mortice of Hospital, Parkville, Victoria, seen post high velocity trauma, it can also present post sorts. Coronally, the cuneiforms are formed in a Australia minor trauma. It is also misdiagnosed in approximately trapezoidal shape and create a ‘Roman Arch’. The ‘ ’ Correspondence to 20% of cases. These Lisfranc injuries typically present to keystone to this is the recessed second metatarsal Dr Simon LauRoyal Melbourne EDs with pain particularly with weight bearing, swelling base. This keystone confers stability in the coronal Hospital, Orthopaedic Office, and post a characteristic mechanism of injury. Diagnosis plane to the midfoot and in cases of Lisfranc frac- Level 7 East, Grattan Street, is via clinical examination and radiological investigation tures dislocation, the loss of the stability and rigid- Parkville VIC 3050, Australia; — [email protected] typically plain radiographs and CTs. Once diagnosed, ity of arch at the midfoot can result in a pes planus Lisfranc injuries can be classified as stable or unstable. -

Residents Comprehensive Fracture Course Faculty: Lunch /Spinesymposium Wednesday - Friday, October 5 - 7
RESIDENTS COMPREHENSIVE FRACTURE COURSE • Wednesday – Friday, Modules Module One October 5 – 7, 2016 Articular Fractures Module Leaders: Frank A. Liporace, MD Toni M. McLaurin, MD & Marcus F. Sciadini, MD, Timothy S. Achor, MD Program Chairs Topics Distal Humerus Course Description Anatomy/Approaches (incl. Osteotomy) Post-Operative Treatment Reduction and Implant Techniques Total Elbow Arthroplasty The Residents Comprehensive Fracture Course will be presented Elbow in six (6) separate small group modules, with 22 - 24 residents Anatomy and Radiographs Olecranon Fractures and 5 experienced faculty educators per module. Monteggia Fractures Terrible Triad Wednesday - Friday, October 5 - 7 - Friday, Wednesday The modules will have a rapid-fire series of mini-lectures, an Tibial Plateau extensive open case-based discussion, video demonstrations of Fragment Specific Fixation Classification techniques, and hands-on skills lab exercises. Modules will cover Medial/Posteromedial Fragment Lateral Articular Fragment fundamental principles of fracture care distributed among six topics: Treatment Algorithms: Low Energy - High Energy articular, diaphyseal, foot & ankle, geriatrics, pediatrics, and Supracondylar Femur pelvis/polytrauma/acetabulum, plus a lunch-time spine session. ORIF - Intramedullary Nailing Anatomy Implant Position and Surgical Techniques Radiography This course offers pre-course educational on-demand video materials. Details will be sent with registration confirmation as Case-Based Discussions well as an online basic science pre-test and an onsite clinical Intra-articular Distal Humerus Fracture Monteggia Fracture post test. Olecranon Fracture Terrible Triad Residents Comprehensive Fracture Course Fracture Residents Comprehensive Intercondylar Femur Fracture Lateral Plateau Fracture Bicondylar Plateau Fracture B3 Distal Femur Fracture Target Audience Hands-on Skills Labs This course is targeted for PGY2 – PGY4’s, others who feel they Olecranon Tension Banding and Plating Tibial Plateau will benefit will not be excluded. -

Lower Extremity Fracture Eponyms (Part 2) Philip Kin-Wai Wong1, Tarek N Hanna2*, Waqas Shuaib3, Stephen M Sanders4 and Faisal Khosa2
Wong et al. International Journal of Emergency Medicine (2015) 8:25 DOI 10.1186/s12245-015-0076-1 REVIEW Open Access What’s in a name? Lower extremity fracture eponyms (Part 2) Philip Kin-Wai Wong1, Tarek N Hanna2*, Waqas Shuaib3, Stephen M Sanders4 and Faisal Khosa2 Abstract Eponymous extremity fractures are commonly encountered in the emergency setting. Correct eponym usage allows rapid, succinct communication of complex injuries. We review both common and less frequently encountered extremity fracture eponyms, focusing on imaging features to identify and differentiate these injuries. We focus on plain radiographic findings, with supporting computed tomography (CT) images. For each injury, important radiologic descriptors are discussed which may need to be communicated to clinicians. Aspects of management and follow-up imaging recommendations are included. This is a two-part review: Part 1 focuses on fracture eponyms of the upper extremity, while Part 2 encompasses fracture eponyms of the lower extremity. Keywords: Eponyms; Fractures; Lower extremities; Imaging Introduction Review: Lower extremity fracture eponyms Eponyms are embedded throughout medicine; they Pipkin fracture can be found in medical literature, textbooks, and Femoral head fractures are relatively uncommon and even mass media. Their use allows physicians to are typically associated with hip dislocations after se- quickly provide a concise description of a complex vere high-impact trauma such as a motor vehicle colli- injury pattern. Eponymous extremity fractures are sion. Femoral head fractures are commonly grouped commonly encountered in the emergency setting and into the Pipkin classification (see Table 1) after the work are frequently used in interactions amongst radiolo- of the orthopedic surgeon Garrett Pipkin in 1957 (Fig. -
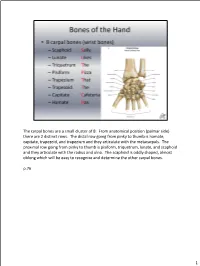
The Carpal Bones Are a Small Cluster of 8. from Anatomical Position (Palmar Side) There Are 2 Distinct Rows
The carpal bones are a small cluster of 8. From anatomical position (palmar side) there are 2 distinct rows. The distal row going from pinky to thumb is hamate, capitate, trapezoid, and trapezium and they articulate with the metacarpals. The proximal row going from pinky to thumb is pisiform, triquetrum, lunate, and scaphoid and they articulate with the radius and ulna. The scaphoid is oddly shaped, almost oblong which will be easy to recognize and determine the other carpal bones. p.76 1 Radiocarpal ligaments (palmar and dorsal)--Connects radius to carpal bones Intercarpal ligaments (palmar and dorsal)--Connects carpal bones to each other Collateral ligaments (ulnar and radial)--Ulnar collateral ligament and radial collateral ligament connect the forearm to the wrist and help support the sides of the wrist as well There are radiocarpal ligaments and intercarpal ligaments on both the dorsal and palmar sides of the hand. p.78 2 So for the middle phalanges there are only #2-5 since the thumb does not have a middle phalange. The proximal phalange and middle phalange articulation is called the proximal interphalangeal joint and the middle phalange articulating with the distal phalange is called the distal interphalangeal joint. With the thumb there is only 1 interphalangeal joint since it’s just the proximal and distal phalange. p.77 3 p.77 4 The radiocarpal joint is where the carpals articulate with the radius (condyloid joint). The intercarpal joints are the gliding joints between the carpals. The 1st carpometacarpal joint is the thumb, which is a saddle joint, but the 2nd-5th carpometacarpal joints are gliding.