Host–Symbiont Specificity Determined by Microbe–Microbe Competition in an Insect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Het News Issue 22 (Spring 2015)
Circulation : An informal newsletter circulated periodically to those interested in Heteroptera Copyright : Text & drawings © 2015 Authors. Photographs © 2015 Photographers Citation : Het News, 3 rd series, 22, Spring 2015 Editor : Tristan Bantock: 101 Crouch Hill, London N8 9RD [email protected] britishbugs.org.uk , twitter.com/BritishBugs CONTENTS ANNOUNCEMENTS Scutelleridae A tribute – Ashley Wood…………………………………………….. 1 Odonotoscelis fuliginosa ……………………………………………... 5 Updated keys to Terrestrial Heteroptera exc. Miridae…………… 2 Stenocephalidae County Recorder News……………………………………………… 2 Dicranocephalus medius feeding on Euphorbia x pseudovirgata 5 IUCN status reviews for Heteroptera………………………………. 2 Lygaeidae New RES Handbook to Shieldbugs & Allies of Britain and Ireland 2 Nysius huttoni ………………………………………………………… 5 Request for photographs of Peribalus spp…………………………. 2 Ortholomus punctipennis …………………….……………………… 5 Ischnodemus sabuleti ……………..………….……………………… 5 SPECIES NEW TO BRITAIN Rhyparochromus vulgaris ……………………………………………. 6 Centrocoris variegatus (Coreidae)………………………………….. 2 Drymus pumilio…………………………………………………….…. 6 Orius horvathi (Anthocoridae)……………………………………….. 2 Miridae Nabis capsiformis (Nabidae)………………………………………… 3 Globiceps fulvicollis cruciatus…………………….………………… 6 Psallus anaemicus (Miridae)………………………………………… 3 Hallodapus montandoni………………………………………………. 6 Psallus helenae (Miridae)……………………………………………. 3 Pachytomella parallela……………………………………………….. 6 Hoplomachus thunbergii……………………………………………… 6 SPECIES NOTES Chlamydatus evanescens……………………… ……………………. -

Biosecurity Plan for the Vegetable Industry
Biosecurity Plan for the Vegetable Industry A shared responsibility between government and industry Version 3.0 May 2018 Plant Health AUSTRALIA Location: Level 1 1 Phipps Close DEAKIN ACT 2600 Phone: +61 2 6215 7700 Fax: +61 2 6260 4321 E-mail: [email protected] Visit our web site: www.planthealthaustralia.com.au An electronic copy of this plan is available through the email address listed above. © Plant Health Australia Limited 2018 Copyright in this publication is owned by Plant Health Australia Limited, except when content has been provided by other contributors, in which case copyright may be owned by another person. With the exception of any material protected by a trade mark, this publication is licensed under a Creative Commons Attribution-No Derivs 3.0 Australia licence. Any use of this publication, other than as authorised under this licence or copyright law, is prohibited. http://creativecommons.org/licenses/by-nd/3.0/ - This details the relevant licence conditions, including the full legal code. This licence allows for redistribution, commercial and non-commercial, as long as it is passed along unchanged and in whole, with credit to Plant Health Australia (as below). In referencing this document, the preferred citation is: Plant Health Australia Ltd (2018) Biosecurity Plan for the Vegetable Industry (Version 3.0 – 2018) Plant Health Australia, Canberra, ACT. This project has been funded by Hort Innovation, using the vegetable research and development levy and contributions from the Australian Government. Hort Innovation is the grower-owned, not for profit research and development corporation for Australian horticulture Disclaimer: The material contained in this publication is produced for general information only. -

Comparative Genomics of Pandoraea, a Genus Enriched in Xenobiotic Biodegradation and Metabolism
fmicb-10-02556 November 4, 2019 Time: 15:40 # 1 ORIGINAL RESEARCH published: 06 November 2019 doi: 10.3389/fmicb.2019.02556 Comparative Genomics of Pandoraea, a Genus Enriched in Xenobiotic Biodegradation and Metabolism Charlotte Peeters1, Evelien De Canck1, Margo Cnockaert1, Evie De Brandt1, Cindy Snauwaert2, Bart Verheyde1, Eliza Depoorter1, Theodore Spilker3, John J. LiPuma3 and Peter Vandamme1,2* 1 Laboratory of Microbiology, Department of Biochemistry and Microbiology, Faculty of Sciences, Ghent University, Ghent, Belgium, 2 BCCM/LMG Bacteria Collection, Department of Biochemistry and Microbiology, Faculty of Sciences, Ghent Edited by: University, Ghent, Belgium, 3 Department of Pediatrics, University of Michigan Medical School, Ann Arbor, MI, United States Iain Sutcliffe, Northumbria University, United Kingdom Comparative analysis of partial gyrB, recA, and gltB gene sequences of 84 Pandoraea Reviewed by: reference strains and field isolates revealed several clusters that included no taxonomic Martin W. Hahn, reference strains. The gyrB, recA, and gltB phylogenetic trees were used to select 27 University of Innsbruck, Austria Stephanus Nicolaas Venter, strains for whole-genome sequence analysis and for a comparative genomics study that University of Pretoria, South Africa also included 41 publicly available Pandoraea genome sequences. The phylogenomic Aharon Oren, The Hebrew University of Jerusalem, analyses included a Genome BLAST Distance Phylogeny approach to calculate pairwise Israel digital DNA–DNA hybridization values and their -

Genomic and Proteomic Analysis of Lignin Degrading and Polyhydroxyalkanoate Accumulating Β‑Proteobacterium Pandoraea Sp
Kumar et al. Biotechnol Biofuels (2018) 11:154 https://doi.org/10.1186/s13068-018-1148-2 Biotechnology for Biofuels RESEARCH Open Access Genomic and proteomic analysis of lignin degrading and polyhydroxyalkanoate accumulating β‑proteobacterium Pandoraea sp. ISTKB Madan Kumar1, Sandhya Verma2, Rajesh Kumar Gazara2, Manish Kumar1, Ashok Pandey3, Praveen Kumar Verma2* and Indu Shekhar Thakur1* Abstract Background: Lignin is a major component of plant biomass and is recalcitrant to degradation due to its complex and heterogeneous aromatic structure. The biomass-based research mainly focuses on polysaccharides component of biomass and lignin is discarded as waste with very limited usage. The sustainability and success of plant polysac- charide-based biorefnery can be possible if lignin is utilized in improved ways and with minimal waste generation. Discovering new microbial strains and understanding their enzyme system for lignin degradation are necessary for its conversion into fuel and chemicals. The Pandoraea sp. ISTKB was previously characterized for lignin degradation and successfully applied for pretreatment of sugarcane bagasse and polyhydroxyalkanoate (PHA) production. In this study, genomic analysis and proteomics on aromatic polymer kraft lignin and vanillic acid are performed to fnd the impor- tant enzymes for polymer utilization. Results: Genomic analysis of Pandoraea sp. ISTKB revealed the presence of strong lignin degradation machinery and identifed various candidate genes responsible for lignin degradation and PHA production. -

(Signoret) (Coreoidea: Stenocephalidae) from Maharashtra State, India
OPEN ACCESS The Journal of Threatened Taxa fs dedfcated to bufldfng evfdence for conservafon globally by publfshfng peer-revfewed arfcles onlfne every month at a reasonably rapfd rate at www.threatenedtaxa.org . All arfcles publfshed fn JoTT are regfstered under Creafve Commons Atrfbufon 4.0 Internafonal Lfcense unless otherwfse menfoned. JoTT allows unrestrfcted use of arfcles fn any medfum, reproducfon, and dfstrfbufon by provfdfng adequate credft to the authors and the source of publfcafon. Journal of Threatened Taxa Bufldfng evfdence for conservafon globally www.threatenedtaxa.org ISSN 0974-7907 (Onlfne) | ISSN 0974-7893 (Prfnt) Communfcatfon Illustrated descrfptfon and notes on bfology of Dfcranocephalus lateralfs (Sfgnoret) (Coreofdea: Stenocephalfdae) from Maharashtra State, Indfa Balasaheb V. Sarode, Nfkhfl U. Joshf, Swapnfl S. Boyane, Subodh S. Gafkwad, Prafk P. Pansare & Hemant V. Ghate 26 October 2017 | Vol. 9| No. 10 | Pp. 10792–10803 10.11609/jot. 3451 .9. 10. 10792-10803 For Focus, Scope, Afms, Polfcfes and Gufdelfnes vfsft htp://threatenedtaxa.org/About_JoTT For Arfcle Submfssfon Gufdelfnes vfsft htp://threatenedtaxa.org/Submfssfon_Gufdelfnes For Polfcfes agafnst Scfenffc Mfsconduct vfsft htp://threatenedtaxa.org/JoTT_Polfcy_agafnst_Scfenffc_Mfsconduct For reprfnts contact <[email protected]> Publfsher/Host Partner Threatened Taxa Journal of Threatened Taxa | www.threatenedtaxa.org | 26 October 2017 | 9(10): 10792–10803 Illustrated description and notes on biology of Communication Dicranocephalus lateralis (Signoret) -

Genomic Plasticity of the Causative Agent of Melioidosis, Burkholderia Pseudomallei
Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei Matthew T. G. Holdena, Richard W. Titballb,c, Sharon J. Peacockd,e, Ana M. Cerden˜ o-Ta´ rragaa, Timothy Atkinsb, Lisa C. Crossmana, Tyrone Pittf, Carol Churchera, Karen Mungalla, Stephen D. Bentleya, Mohammed Sebaihiaa, Nicholas R. Thomsona, Nathalie Basona, Ifor R. Beachamg, Karen Brooksa, Katherine A. Brownh, Nat F. Browng, Greg L. Challisi, Inna Cherevacha, Tracy Chillingwortha, Ann Cronina, Ben Crossetth, Paul Davisa, David DeShazerj, Theresa Feltwella, Audrey Frasera, Zahra Hancea, Heidi Hausera, Simon Holroyda, Kay Jagelsa, Karen E. Keithh, Mark Maddisona, Sharon Moulea, Claire Pricea, Michael A. Quaila, Ester Rabbinowitscha, Kim Rutherforda, Mandy Sandersa, Mark Simmondsa, Sirirurg Songsivilaik, Kim Stevensa, Sarinna Tumapae, Monkgol Vesaratchaveste, Sally Whiteheada, Corin Yeatsa, Bart G. Barrella, Petra C. F. Oystonb, and Julian Parkhilla,l aWellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SA, United Kingdom; bDefence Science and Technology Laboratory, Porton Down, Salisbury SP4 0JQ, United Kingdom; cDepartment of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London WC1E 7HT, United Kingdom; dNuffield Department of Clinical Medicine, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, United Kingdom; eFaculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand; fLaboratory of Hospital Infection, Division of Nosocomial Infection Prevention and Control, Central Public Health Laboratory, London NW9 5HT, United Kingdom; gSchool of Health Science, Griffith University, Gold Coast, Queensland 9726, Australia; hDepartment of Biological Sciences, Centre for Molecular Microbiology and Infection, Flowers Building, Imperial College, London SW7 2AZ, United Kingdom; iDepartment of Chemistry, University of Warwick, Coventry CV4 7AL, United Kingdom; jU.S. -

Broad-Headed Bugs (Alydidae)
Chapter 18 Broad-Headed Bugs (Alydidae) Antônio R. Panizzi and Carl w. Schaefer Abstract The broad-headed bugs (Alydidae) are divided into two subfamilies, Alydinaeand Micrelytrinae, each divided into two tribes, Daclerini and Alydini, and Micrelytriniand Leptocorisini, respectively, The farnily has 53 genera and about 250 specieins; the Neotropics, there are 21 genera. Alydids are small (8-20 mm), slen- Itr,with a triangular head; nymphs of alydines mimic ants, the adults of some Micrelytrinialso rnirnic ants. The most studied species in the Neotropics is the aly- dineNeomegalotomus parvus (Westwood), usually associated with legumes, and maybe a pest on soybean. Other common genera include Hyalymenus Amyot & Serville,Stenocoris Burmeister, Cydamus Stâl, and Trachelium Herrich-Schâffer. Studieson taxonomy and bioecology on alydids of the Neotropics are needed. 18.1 Introduction AlydidaeAmyot and Serville, 1843, were treated as a subfarnily of the farnily Coreidaeand even as a tribe (Schaffner 1964); now it has been treated as a farnily, ~ether with Coreidae, Rhopalidae, Hyocephalidae, and Stenocephalidae, in the !UperfarniCoreoidealy (Schaefer 1964). Thisfarnily contains 53 genera and approximately 250 species, mostly tropical Irsubtropical,in all regions of the world. There are only two genera that span both dleOldand the New World, Alydus and Megalotomus. These genera are Holarctic, IInAlydus extends from Alaska through Canada into Mexico (Brailovsky and Flores 1979;Froeschner 1988; Maw et al. 2000). The genera of Alydinae have been revised by Schaffner (1964; 22 species worldwide);the world genera of the subfamily Micrelytrinae, tribe Leptocorisini, were CarlW.Schaefer: Author deceased at the time of publication A.RP.anizzi ([gJ) Laboratóriode Entomologia, Embrapa Trigo, Caixa Postal 3081, Passo Fundo, RS9900l-970,Brazil e-mail:[email protected] eSpringerScience-Business Media Dordrecht 2015 537 :I.R.Panizzi,J. -

Synopsis of the Heteroptera Or True Bugs of the Galapagos Islands
Synopsis of the Heteroptera or True Bugs of the Galapagos Islands ' 4k. RICHARD C. JROESCHNE,RD SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 407 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Characterization of Bacterial Communities Associated
www.nature.com/scientificreports OPEN Characterization of bacterial communities associated with blood‑fed and starved tropical bed bugs, Cimex hemipterus (F.) (Hemiptera): a high throughput metabarcoding analysis Li Lim & Abdul Hafz Ab Majid* With the development of new metagenomic techniques, the microbial community structure of common bed bugs, Cimex lectularius, is well‑studied, while information regarding the constituents of the bacterial communities associated with tropical bed bugs, Cimex hemipterus, is lacking. In this study, the bacteria communities in the blood‑fed and starved tropical bed bugs were analysed and characterized by amplifying the v3‑v4 hypervariable region of the 16S rRNA gene region, followed by MiSeq Illumina sequencing. Across all samples, Proteobacteria made up more than 99% of the microbial community. An alpha‑proteobacterium Wolbachia and gamma‑proteobacterium, including Dickeya chrysanthemi and Pseudomonas, were the dominant OTUs at the genus level. Although the dominant OTUs of bacterial communities of blood‑fed and starved bed bugs were the same, bacterial genera present in lower numbers were varied. The bacteria load in starved bed bugs was also higher than blood‑fed bed bugs. Cimex hemipterus Fabricus (Hemiptera), also known as tropical bed bugs, is an obligate blood-feeding insect throughout their entire developmental cycle, has made a recent resurgence probably due to increased worldwide travel, climate change, and resistance to insecticides1–3. Distribution of tropical bed bugs is inclined to tropical regions, and infestation usually occurs in human dwellings such as dormitories and hotels 1,2. Bed bugs are a nuisance pest to humans as people that are bitten by this insect may experience allergic reactions, iron defciency, and secondary bacterial infection from bite sores4,5. -

Coastal Vegetated Shingle
Natural England Commissioned Report NECR054 Coastal Vegetated Shingle Development of an evidence base of the extent and quality of shingle habitats in England to improve targeting and delivery of the coastal vegetated shingle HAP First published 17 December 2010 www.naturalengland.org.uk Foreword Natural England commission a range of reports from external contractors to provide evidence and advice to assist us in delivering our duties. This work was jointly funded by the National Trust, Defra and managed by Natural England with support of a project steering group. The views in this report are those of the authors and do not necessarily represent those of Natural England. Background Vegetated shingle is a Biodiversity Action Plan assessment, especially related to long-term climate priority habitat because it is so rare and so valuable change and sea level rise. for wildlife. All the major examples of the habitat and The data and other products will also be used by many of the minor ones have been notified for their Natural England and partner organisations in other wildlife value. To help identify restoration targets and contexts, such as the evaluation of shingle resources monitor the habitat we need to know what there is, within flood risk management applications; where it is, its geomorphology and the activities incorporating the scales of change that have been taking place that could affect it. observed and allowing assessment of options for This study was commissioned to provide a spatial longer term adaptation to climate change. Whilst dataset of the inventory for coastal vegetated shingle recognising the limitations of the work, this will inform in England. -

Genomic Comparison of Insect Gut Symbionts from Divergent Burkholderia Subclades
G C A T T A C G G C A T genes Article Genomic Comparison of Insect Gut Symbionts from Divergent Burkholderia Subclades Kazutaka Takeshita 1,* and Yoshitomo Kikuchi 2,3 1 Faculty of Bioresource Sciences, Akita Prefectural University, Akita City, Akita 010-0195, Japan 2 Bioproduction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Hokkaido Center, Sapporo, Hokkaido 062-8517, Japan; [email protected] 3 Graduate School of Agriculture, Hokkaido University, Sapporo, Hokkaido 060-8589, Japan * Correspondence: [email protected] Received: 10 June 2020; Accepted: 1 July 2020; Published: 3 July 2020 Abstract: Stink bugs of the superfamilies Coreoidea and Lygaeoidea establish gut symbioses with environmentally acquired bacteria of the genus Burkholderia sensu lato. In the genus Burkholderia, the stink bug-associated strains form a monophyletic clade, named stink bug-associated beneficial and environmental (SBE) clade (or Caballeronia). Recently, we revealed that members of the family Largidae of the superfamily Pyrrhocoroidea are associated with Burkholderia but not specifically with the SBE Burkholderia; largid bugs harbor symbionts that belong to a clade of plant-associated group of Burkholderia, called plant-associated beneficial and environmental (PBE) clade (or Paraburkholderia). To understand the genomic features of Burkholderia symbionts of stink bugs, we isolated two symbiotic Burkholderia strains from a bordered plant bug Physopellta gutta (Pyrrhocoroidea: Largidae) and determined their complete genomes. The genome sizes of the insect-associated PBE (iPBE) are 9.5 Mb and 11.2 Mb, both of which are larger than the genomes of the SBE Burkholderia symbionts. A whole-genome comparison between two iPBE symbionts and three SBE symbionts highlighted that all previously reported symbiosis factors are shared and that 282 genes are specifically conserved in the five stink bug symbionts, over one-third of which have unknown function. -
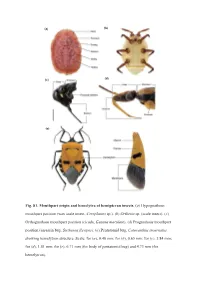
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V