The Migration of Paraxial and Lateral Plate Mesoderm Cells Emerging from the Late Primitive Streak Is Controlled by Different Wnt Signals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

The Zebrafish Presomitic Mesoderm Elongates Through Compression-Extension
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.11.434927; this version posted March 11, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The zebrafish presomitic mesoderm elongates through compression-extension Lewis Thomson1, Leila Muresan2 and Benjamin Steventon1* 1) Department of Genetics, University of Cambridge, Cambridge, UK, CB2 3EH 2) Cambridge Advanced Imaging Centre, University of Cambridge, Cambridge, UK *[email protected] Abstract In vertebrate embryos the presomitic mesoderm become progressively segmented into somites at the anterior end while extending along the anterior- posterior axis. A commonly adopted model to explain how this tissue elongates is that of posterior growth, driven in part by the addition of new cells from uncommitted progenitor populations in the tailbud. However, in zebrafish, much of somitogenesis is associated with an absence of overall volume increase and posterior progenitors do not contribute new cells until the final stages of somitogenesis. Here, we perform a comprehensive 3D morphometric analysis of the paraxial mesoderm and reveal that extension is linked to a volumetric decrease, compression in both dorsal-ventral and medio-lateral axes, and an increase in cell density. We also find that individual cells decrease in their cell volume over successive somite stages. Live cell tracking confirms that much of this tissue deformation occurs within the presomitic mesoderm progenitor zone and is associated with non-directional rearrangement. Furthermore, unlike the trunk somites that are laid down during gastrulation, tail somites develop from a tissue that can continue to elongate in the absence of functional PCP signalling. -

From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal Interactions During Embryonic Development
International Journal of Molecular Sciences Review From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal Interactions during Embryonic Development Nitza Kahane and Chaya Kalcheim * Department of Medical Neurobiology, Institute of Medical Research Israel-Canada (IMRIC) and the Edmond and Lily Safra Center for Brain Sciences (ELSC), Hebrew University of Jerusalem-Hadassah Medical School, P.O. Box 12272, Jerusalem 9112102, Israel; [email protected] * Correspondence: [email protected] Abstract: To ensure the formation of a properly patterned embryo, multiple processes must operate harmoniously at sequential phases of development. This is implemented by mutual interactions between cells and tissues that together regulate the segregation and specification of cells, their growth and morphogenesis. The formation of the spinal cord and paraxial mesoderm derivatives exquisitely illustrate these processes. Following early gastrulation, while the vertebrate body elongates, a pop- ulation of bipotent neuromesodermal progenitors resident in the posterior region of the embryo generate both neural and mesodermal lineages. At later stages, the somitic mesoderm regulates aspects of neural patterning and differentiation of both central and peripheral neural progenitors. Reciprocally, neural precursors influence the paraxial mesoderm to regulate somite-derived myogen- esis and additional processes by distinct mechanisms. Central to this crosstalk is the activity of the axial notochord, which, via sonic hedgehog signaling, plays pivotal roles in neural, skeletal muscle and cartilage ontogeny. Here, we discuss the cellular and molecular basis underlying this complex Citation: Kahane, N.; Kalcheim, C. developmental plan, with a focus on the logic of sonic hedgehog activities in the coordination of the From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal neural-mesodermal axis. -

Comparing the Differentiation Potential of Brachyury+ Mesodermal Cells
bioRxiv preprint doi: https://doi.org/10.1101/239913; this version posted December 26, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 of 44 + 1 Comparing the differentiation potential of Brachyury mesodermal 2 cells generated from 3-D and 2-D culture systems 3 Jing Zhou 1, Antonius Plagge 1 and Patricia Murray 1,* 4 1 Institute of Translational Medicine, University of Liverpool, Liverpool L69 3BX, UK; E-mails: 5 [email protected] (J.Z.); [email protected] (A.P.); [email protected] 6 (P.M.) 7 * Correspondence: [email protected] 8 Abstract 9 Mesodermal populations can be generated in vitro from mouse embryonic stem cells (mESCs) using 10 three-dimensional (3-D) aggregates called embryoid bodies or two-dimensional (2-D) monolayer 11 culture systems. Here, we investigated whether Brachyury-expressing mesodermal cells generated 12 using 3-D or 2-D culture systems are equivalent, or instead, have different properties. Using a 13 Brachyury-GFP/E2-Crimson reporter mESC line, we isolated Brachyury-GFP+ mesoderm cells using 14 flow-activated cell sorting and compared their gene expression profiles and ex vivo differentiation 15 patterns. Quantitative RT-PCR analysis showed significant up-regulation of Cdx2, Foxf1 and Hoxb1 in 16 the Brachyury-GFP+ cells isolated from the 3-D system compared with those isolated from the 2-D 17 system. -

The Genetic Basis of Mammalian Neurulation
REVIEWS THE GENETIC BASIS OF MAMMALIAN NEURULATION Andrew J. Copp*, Nicholas D. E. Greene* and Jennifer N. Murdoch‡ More than 80 mutant mouse genes disrupt neurulation and allow an in-depth analysis of the underlying developmental mechanisms. Although many of the genetic mutants have been studied in only rudimentary detail, several molecular pathways can already be identified as crucial for normal neurulation. These include the planar cell-polarity pathway, which is required for the initiation of neural tube closure, and the sonic hedgehog signalling pathway that regulates neural plate bending. Mutant mice also offer an opportunity to unravel the mechanisms by which folic acid prevents neural tube defects, and to develop new therapies for folate-resistant defects. 6 ECTODERM Neurulation is a fundamental event of embryogenesis distinct locations in the brain and spinal cord .By The outer of the three that culminates in the formation of the neural tube, contrast, the mechanisms that underlie the forma- embryonic (germ) layers that which is the precursor of the brain and spinal cord. A tion, elevation and fusion of the neural folds have gives rise to the entire central region of specialized dorsal ECTODERM, the neural plate, remained elusive. nervous system, plus other organs and embryonic develops bilateral neural folds at its junction with sur- An opportunity has now arisen for an incisive analy- structures. face (non-neural) ectoderm. These folds elevate, come sis of neurulation mechanisms using the growing battery into contact (appose) in the midline and fuse to create of genetically targeted and other mutant mouse strains NEURAL CREST the neural tube, which, thereafter, becomes covered by in which NTDs form part of the mutant phenotype7.At A migratory cell population that future epidermal ectoderm. -

Understanding Paraxial Mesoderm Development and Sclerotome Specification for Skeletal Repair Shoichiro Tani 1,2, Ung-Il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3
Tani et al. Experimental & Molecular Medicine (2020) 52:1166–1177 https://doi.org/10.1038/s12276-020-0482-1 Experimental & Molecular Medicine REVIEW ARTICLE Open Access Understanding paraxial mesoderm development and sclerotome specification for skeletal repair Shoichiro Tani 1,2, Ung-il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3 Abstract Pluripotent stem cells (PSCs) are attractive regenerative therapy tools for skeletal tissues. However, a deep understanding of skeletal development is required in order to model this development with PSCs, and for the application of PSCs in clinical settings. Skeletal tissues originate from three types of cell populations: the paraxial mesoderm, lateral plate mesoderm, and neural crest. The paraxial mesoderm gives rise to the sclerotome mainly through somitogenesis. In this process, key developmental processes, including initiation of the segmentation clock, formation of the determination front, and the mesenchymal–epithelial transition, are sequentially coordinated. The sclerotome further forms vertebral columns and contributes to various other tissues, such as tendons, vessels (including the dorsal aorta), and even meninges. To understand the molecular mechanisms underlying these developmental processes, extensive studies have been conducted. These studies have demonstrated that a gradient of activities involving multiple signaling pathways specify the embryonic axis and induce cell-type-specific master transcription factors in a spatiotemporal manner. Moreover, applying the knowledge of mesoderm development, researchers have attempted to recapitulate the in vivo development processes in in vitro settings, using mouse and human PSCs. In this review, we summarize the state-of-the-art understanding of mesoderm development and in vitro modeling of mesoderm development using PSCs. We also discuss future perspectives on the use of PSCs to generate skeletal tissues for basic research and clinical applications. -

The Derivatives of Three-Layered Embryo (Germ Layers)
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FourthFourth week:week: TheThe derivativesderivatives ofof trilaminartrilaminar germgerm discdisc Dorsal side of the germ disc. At the beginning of the third week of development, the ectodermal germ layer has the shape of a disc that is broader in the cephalic than the caudal region. Cross section shows formation of trilaminar germ disc Primitive pit Drawing of a sagittal section through a 17-day embryo. The most cranial portion of the definitive notochord has formed. ectoderm Schematic view showing the definitive notochord. horizon =ectoderm hillside fields =neural plate mountain peaks =neural folds Cave sinks into mountain =neural tube valley =neural groove 7.1 Derivatives of the Ectodermal Germ Layer 1) Formation of neural tube Notochord induces the overlying ectoderm to thicken and form the neural plate. Cross section Animation of formation of neural plate When notochord is forming, primitive streak is shorten. At meanwhile, neural plate is induced to form cephalic to caudal end, following formation of notochord. By the end of 3rd week, neural folds and neural groove are formed. Neural folds fuse in the midline, beginning in cervical region and Cross section proceeding cranially and caudally. Neural tube is formed & invade into the embryo body. A. Dorsal view of a human embryo at approximately day 22. B. Dorsal view of a human embryo at approximately day 23. The nervous system is in connection with the amniotic cavity through the cranial and caudal neuropores. Cranial/anterior neuropore Neural fold heart Neural groove endoderm caudal/posterior neuropore A. -

Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos
Review Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos Jörg Männer 1,*,† and Talat Mesud Yelbuz 2,† 1 Group Cardio‐Embryology, Institute of Anatomy and Embryology UMG, Georg‐August‐University Goettingen, D‐37075 Goettingen, Germany; [email protected] 2 Department of Cardiac Sciences, King Abdulaziz Cardiac Center, Section of Pediatric Cardiology, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh 11426, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.: +49‐551‐39‐7032 † This work is dedicated to the memory of our academic mentors Gerd Steding (1936–2011) and Armin Wessel (1946–2011). Received: 29 January 2019; Accepted: 21 February 2019; Published: 27 February 2019 Abstract: The early embryonic heart is a multi‐layered tube consisting of (1) an outer myocardial tube; (2) an inner endocardial tube; and (3) an extracellular matrix layer interposed between the myocardium and endocardium, called “cardiac jelly” (CJ). During the past decades, research on CJ has mainly focused on its molecular and cellular biological aspects. This review focuses on the morphological and biomechanical aspects of CJ. Special attention is given to (1) the spatial distribution and fiber architecture of CJ; (2) the morphological dynamics of CJ during the cardiac cycle; and (3) the removal/remodeling of CJ during advanced heart looping stages, which leads to the formation of ventricular trabeculations and endocardial cushions. CJ acts as a hydraulic skeleton, displaying striking structural and functional similarities with the mesoglea of jellyfish. CJ not only represents a filler substance, facilitating end‐systolic occlusion of the embryonic heart lumen. -
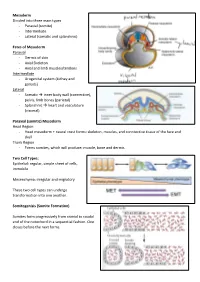
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

12. Development of Axial Skeleton and Extremities. Muscles and Skin
Z. Tonar, M. Králíčková: Outlines of lectures on embryology for 2 nd year students of General medicine and Dentistry License Creative Commons - http://creativecommons.org/licenses/by-nc-nd/3.0/ 12. Development of axial skeleton and extremities. Muscles and skin. Timeline − 19 days: somites emerge in the gastrula − 4 weeks: sclerotome cells migrate along the neural tube − 5 weeks: mesenchymal blastema of the axial skeleton − 6 weeks: mesenchymal blastema of limbs − 8 weeks: rotation of the limbs − 8 weeks: individual muscles differentiate − 10 weeks: primary ossification centres in diaphyses − 3 months: bones of the skull develop − 9 months: diaphyses ossified; secondary ossification centres emerge In general, bone tissue originates from: − the of the somitic paraxial mesoderm, namely from the ventromedial part, the sclerotome − the head non-segmented mesoderm − the somatopleuric lateral plate mesoderm skeleton of limbs − the neural crest, which differentiates into the head ectomesenchyme − mesenchyme o its cells migrate and differentiate into fibroblasts, the source of the desmogenous (intramembranous) ossification o its cells imgrate and differentiate into chondroblasts, the source of the chondrogenous ossification of the hyaline cartilage models Limbs − week 4: limb buds o somatopleuric mesenchyme differentiates into bones and connective tissues o myogenic cells, angioblasts and nerves grow in o surface ectoderm thickens into the apical ectodermal ridge − week 6: each limb is divided proximodistally into three components: o autopod -

The Dorsal Neural Tube Organizes the Dermamyotome and Induces Axial Myocytes in the Avian Embryo
Development 122, 231-241 (1996) 231 Printed in Great Britain © The Company of Biologists Limited 1996 DEV3253 The dorsal neural tube organizes the dermamyotome and induces axial myocytes in the avian embryo Martha S. Spence1, Joseph Yip2 and Carol A. Erickson1,* 1Section of Molecular and Cellular Biology, University of California, Davis, CA 95616, USA 2Department of Neurobiology, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA *Author for correspondence (e-mail: [email protected]) SUMMARY Somites, like all axial structures, display dorsoventral reported previously to induce sclerotome. Thus, we have polarity. The dorsal portion of the somite forms the der- demonstrated that in the context of the embryonic envi- mamyotome, which gives rise to the dermis and axial mus- ronment, a dorsalizing signal from the dorsal neural tube culature, whereas the ventromedial somite disperses to can compete with the diffusible ventralizing signal from the generate the sclerotome, which later comprises the notochord. vertebrae and intervertebral discs. Although the neural In contrast to dorsal neural tube, pieces of ventral neural tube and notochord are known to regulate some aspects of tube, dorsal ectoderm or neural crest cells, all of which this dorsoventral pattern, the precise tissues that initially have been postulated to control dermamyotome formation specify the dermamyotome, and later the myotome from it, or to induce myogenesis, either fail to do so or provoke only have been controversial. Indeed, dorsal and ventral neural minimal inductive responses in any of our assays. However, tube, notochord, ectoderm and neural crest cells have all complicating the issue, we find consistent with previous been proposed to influence dermamyotome formation or to studies that following ablation of the entire neural tube, regulate myocyte differentiation.