12. Development of Axial Skeleton and Extremities. Muscles and Skin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

The Zebrafish Presomitic Mesoderm Elongates Through Compression-Extension
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.11.434927; this version posted March 11, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The zebrafish presomitic mesoderm elongates through compression-extension Lewis Thomson1, Leila Muresan2 and Benjamin Steventon1* 1) Department of Genetics, University of Cambridge, Cambridge, UK, CB2 3EH 2) Cambridge Advanced Imaging Centre, University of Cambridge, Cambridge, UK *[email protected] Abstract In vertebrate embryos the presomitic mesoderm become progressively segmented into somites at the anterior end while extending along the anterior- posterior axis. A commonly adopted model to explain how this tissue elongates is that of posterior growth, driven in part by the addition of new cells from uncommitted progenitor populations in the tailbud. However, in zebrafish, much of somitogenesis is associated with an absence of overall volume increase and posterior progenitors do not contribute new cells until the final stages of somitogenesis. Here, we perform a comprehensive 3D morphometric analysis of the paraxial mesoderm and reveal that extension is linked to a volumetric decrease, compression in both dorsal-ventral and medio-lateral axes, and an increase in cell density. We also find that individual cells decrease in their cell volume over successive somite stages. Live cell tracking confirms that much of this tissue deformation occurs within the presomitic mesoderm progenitor zone and is associated with non-directional rearrangement. Furthermore, unlike the trunk somites that are laid down during gastrulation, tail somites develop from a tissue that can continue to elongate in the absence of functional PCP signalling. -

From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal Interactions During Embryonic Development
International Journal of Molecular Sciences Review From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal Interactions during Embryonic Development Nitza Kahane and Chaya Kalcheim * Department of Medical Neurobiology, Institute of Medical Research Israel-Canada (IMRIC) and the Edmond and Lily Safra Center for Brain Sciences (ELSC), Hebrew University of Jerusalem-Hadassah Medical School, P.O. Box 12272, Jerusalem 9112102, Israel; [email protected] * Correspondence: [email protected] Abstract: To ensure the formation of a properly patterned embryo, multiple processes must operate harmoniously at sequential phases of development. This is implemented by mutual interactions between cells and tissues that together regulate the segregation and specification of cells, their growth and morphogenesis. The formation of the spinal cord and paraxial mesoderm derivatives exquisitely illustrate these processes. Following early gastrulation, while the vertebrate body elongates, a pop- ulation of bipotent neuromesodermal progenitors resident in the posterior region of the embryo generate both neural and mesodermal lineages. At later stages, the somitic mesoderm regulates aspects of neural patterning and differentiation of both central and peripheral neural progenitors. Reciprocally, neural precursors influence the paraxial mesoderm to regulate somite-derived myogen- esis and additional processes by distinct mechanisms. Central to this crosstalk is the activity of the axial notochord, which, via sonic hedgehog signaling, plays pivotal roles in neural, skeletal muscle and cartilage ontogeny. Here, we discuss the cellular and molecular basis underlying this complex Citation: Kahane, N.; Kalcheim, C. developmental plan, with a focus on the logic of sonic hedgehog activities in the coordination of the From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal neural-mesodermal axis. -

Polythelia -Six Nipples in Amiddle Varun Arunagiri in the Accessory Nipples
Stanley Medical Journal CASE REPORT - GENERAL SURGERY Polythelia - Six Nipples in a middle aged woman Varun Arunagiri(1), Kothai Anbalagan(1) Abstract Vol 3 | Issue 2 | April - June | 2016 - June 2 | April 3 | Issue Vol Supernumerary nipples are more than two nipples which normally exist in humans. Polythelia or supernumerary nipple is a rare condition with higher prevalence in males than in females with the ratio of 1.7:1. The maximum reported number of nipples in a person with Polythelia is seven in a male. The usual presentation of Polythelia is with three nipples. Here is an image of Polythelia in a 40 year old female presenting with six nipples without lactation from the supernumer- ary nipples and any other anomalies. She has breast fed her two children. Key-words: Polythelia; Supernumerary Nipples; Mammary ridge; Kajava Classification; Clear cells of Toker. Key Messages: 1. Polythelia is a benign condition with chances of malignancy in the accessory nipples. 2. Constant follow-up is needed when the patient says lump in the region of accessory nipple. 3. Lactation during the pregnancy is common. INTRODUCTION: reported number of nipples in a person with Polythelia is seven in a male. The usual presentation of polythelia is with Polythelia is a congenital anomaly of the breast three nipples where in there are accessory nipples along the milk line Mammals have six to seven nipples, which are apart from the normal two nipples. Amazia, polymazia, common among canines and felines. It is rare to see humans Polythelia, athelia are few congenital anomalies of the nip- with more than three nipples. -

The Genetic Basis of Mammalian Neurulation
REVIEWS THE GENETIC BASIS OF MAMMALIAN NEURULATION Andrew J. Copp*, Nicholas D. E. Greene* and Jennifer N. Murdoch‡ More than 80 mutant mouse genes disrupt neurulation and allow an in-depth analysis of the underlying developmental mechanisms. Although many of the genetic mutants have been studied in only rudimentary detail, several molecular pathways can already be identified as crucial for normal neurulation. These include the planar cell-polarity pathway, which is required for the initiation of neural tube closure, and the sonic hedgehog signalling pathway that regulates neural plate bending. Mutant mice also offer an opportunity to unravel the mechanisms by which folic acid prevents neural tube defects, and to develop new therapies for folate-resistant defects. 6 ECTODERM Neurulation is a fundamental event of embryogenesis distinct locations in the brain and spinal cord .By The outer of the three that culminates in the formation of the neural tube, contrast, the mechanisms that underlie the forma- embryonic (germ) layers that which is the precursor of the brain and spinal cord. A tion, elevation and fusion of the neural folds have gives rise to the entire central region of specialized dorsal ECTODERM, the neural plate, remained elusive. nervous system, plus other organs and embryonic develops bilateral neural folds at its junction with sur- An opportunity has now arisen for an incisive analy- structures. face (non-neural) ectoderm. These folds elevate, come sis of neurulation mechanisms using the growing battery into contact (appose) in the midline and fuse to create of genetically targeted and other mutant mouse strains NEURAL CREST the neural tube, which, thereafter, becomes covered by in which NTDs form part of the mutant phenotype7.At A migratory cell population that future epidermal ectoderm. -

Understanding Paraxial Mesoderm Development and Sclerotome Specification for Skeletal Repair Shoichiro Tani 1,2, Ung-Il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3
Tani et al. Experimental & Molecular Medicine (2020) 52:1166–1177 https://doi.org/10.1038/s12276-020-0482-1 Experimental & Molecular Medicine REVIEW ARTICLE Open Access Understanding paraxial mesoderm development and sclerotome specification for skeletal repair Shoichiro Tani 1,2, Ung-il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3 Abstract Pluripotent stem cells (PSCs) are attractive regenerative therapy tools for skeletal tissues. However, a deep understanding of skeletal development is required in order to model this development with PSCs, and for the application of PSCs in clinical settings. Skeletal tissues originate from three types of cell populations: the paraxial mesoderm, lateral plate mesoderm, and neural crest. The paraxial mesoderm gives rise to the sclerotome mainly through somitogenesis. In this process, key developmental processes, including initiation of the segmentation clock, formation of the determination front, and the mesenchymal–epithelial transition, are sequentially coordinated. The sclerotome further forms vertebral columns and contributes to various other tissues, such as tendons, vessels (including the dorsal aorta), and even meninges. To understand the molecular mechanisms underlying these developmental processes, extensive studies have been conducted. These studies have demonstrated that a gradient of activities involving multiple signaling pathways specify the embryonic axis and induce cell-type-specific master transcription factors in a spatiotemporal manner. Moreover, applying the knowledge of mesoderm development, researchers have attempted to recapitulate the in vivo development processes in in vitro settings, using mouse and human PSCs. In this review, we summarize the state-of-the-art understanding of mesoderm development and in vitro modeling of mesoderm development using PSCs. We also discuss future perspectives on the use of PSCs to generate skeletal tissues for basic research and clinical applications. -

A Study of Evaluation and Management of Rare Congenital Breast Diseases Surgery Section
Original Article DOI: 10.7860/JCDR/2016/21077.8648 A Study of Evaluation and Management of Rare Congenital Breast Diseases Surgery Section RIKKI SINGAL1, SUDHIR KUMAR MEHTA2, JYOTI BALA3, MUZZAFAR ZAMAN4, AMIT MITTAL5, GUARAV GUPTA6, SAMER RUDRA7, SAMITA SINGAL8 ABSTRACT Results: Out of 32 cases: 1(3.125%) male patient had Introduction: Polymastia and polythelia may be asymptomatic unilateral and 1(3.125%) male had bilateral accessory nipple, or cause pain, restriction of arm movement, milk discharge, 7 (21.87%) females had unilateral and 1(3.125%) had bilateral cosmetic problems or anxiety. Cosmesis is the main indication accessory nipple, 1 (3.125%) diagnosed as accessory axillary for surgical excision of accessory breasts in axilla. In addition fibroadenoma in female, 16(50%) presented as unilateral and 5 it also confirms the diagnosis and allays the patient’s fear of (15.62%) had bilateral swelling in the axilla as accessory breast. harbouring a malignancy. Patients underwent surgical excision and in 8(25%) cases z- shaped incision was made in view of better cosmesis. Patients Aim: To evaluate the presentation of symptoms, investigations were followed up upto 6 months postoperatively. There were no required for diagnosis and the management to improve the residual swelling and movements of the arm over the shoulder treatment protocols in patients with breast diseases. joint were normal. In 3(9.37%) cases, wound dehiscence Materials and Methods: This retrospective study on breast occurred; in 2 (6.25%) cases lymphoedema formation was diseases presenting as supernumerary breasts and nipples seen. These were successfully managed conservatively. was conducted in the Department of Surgery between January Conclusion: As breast swellings either fibroadenoma or 2013 and January 2016 at MMIMS Research and hospital, carcinoma are common entities to come across everywhere Mullana, Ambala. -

Diagnosis and Treatment of Accessory Breast Cancer in 11 Patients
ONCOLOGY LETTERS 10: 1783-1788, 2015 Diagnosis and treatment of accessory breast cancer in 11 patients SHUO ZHANG1,2*, YONG-HUA YU2, WEI QU2, YONG ZHANG2* and JIA LI2 1School of Medical and Life Sciences, Shandong Academy of Medical Sciences, Jinan University, Jinan, Shandong 250200; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Jinan, Shandong 250117, P.R. China Received August 21, 2014; Accepted May 8, 2015 DOI: 10.3892/ol.2015.3388 Abstract. The present study aimed to investigate the clinical from the ectodermal ridges, also known as the milk lines, on characteristics, diagnosis and treatment of accessory breast the ventral surface of the body, which extend from the axillae cancer, and contribute valuable information regarding this to the inguinal regions and end on the medial aspect of the rare tumour to the current literature, ultimately facilitating thighs on each side of the body (4). Embryologically, ectopic the development of improved treatment strategies. The present breast tissue develops as a result of failed resolution of the study reported the cases of 11 patients with accessory breast mammary ridge, an ectodermal thickening that extends from cancer. The patients with accessory breast cancer were the axilla to the groin (5). Ectopic breast tissue may appear at admitted between January 2002 and June 2014, and the patient any site along the milk line, but it occurs most commonly in records were retrospectively analysed. All patients presented the axill; less commonly, it may appear in locations outside with a tumour that was localised in the axilla. Out of these of the mammary ridge, including the face, middle back, patients, there were 8 patients with invasive ductal carcinoma buttock, posterior neck, chest, vulva, hip, posterior, flank and 3 patients with invasive lobular carcinoma. -
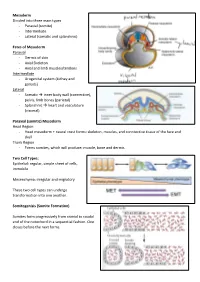
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -
Anatomy of the Breast Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of the Breast Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives By the end of the lecture, the student should be able to: ✓ Describe the shape and position of the female breast. ✓ Describe the structure of the mammary gland. ✓ List the blood supply of the female breast. ✓ Describe the lymphatic drainage of the female breast. ✓ Describe the applied anatomy in the female breast. Highly recommended Introduction 06:26 Overview of the breast: • The breast (consists of mammary glands + associated skin & Extra connective tissue) is a gland made up of lobes arranged radially .around the nipple (شعاعيا) • Each lobe is further divided into lobules. Between the lobes and lobules we have fat & ligaments called ligaments of cooper • These ligaments attach the skin to the muscle (beneath the breast) to give support to the breast. in shape (مخروطي) *o Shape: it is conical o Position: It lies in superficial fascia of the front of chest. * o Parts: It has a: 1. Base lies on muscles, (حلمة الثدي) Apex nipple .2 3. Tail extend into axilla Extra Position of Female Breast (حلقة ملونة) Base Nipple Areola o Extends from 2nd to 6th ribs. o It extends from the lateral margin of sternum medially to the midaxillary line laterally. o It has no capsule. o It lies on 3 muscles: • 2/3 of its base on (1) pectoralis major* Extra muscle, • inferolateral 1/3 on (2) Serratus anterior & (3) External oblique muscles (muscle of anterior abdominal wall). o Its superolateral part sends a process into the axilla called the axillary tail or axillary process. -

The Migration of Paraxial and Lateral Plate Mesoderm Cells Emerging from the Late Primitive Streak Is Controlled by Different Wnt Signals
The Migration of Paraxial and Lateral Plate Mesoderm Cells Emerging from the Late Primitive Streak is Controlled by Different Wnt Signals The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Sweetman, Dylan, Laura Wagstaff, Oliver Cooper, Cornelis Weijer, and Andrea Münsterberg. 2008. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Developmental Biology 8: 63. Published Version doi:10.1186/1471-213X-8-63 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:4774184 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA BMC Developmental Biology BioMed Central Research article Open Access The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals Dylan Sweetman1, Laura Wagstaff2, Oliver Cooper1,3, Cornelis Weijer4 and Andrea Münsterberg*1 Address: 1School of Biological Sciences, University of East Anglia, Norwich Research Park, Norwich, NR4 7TJ, UK, 2Biomedical Research Centre, University of East Anglia, Norwich Research Park, Norwich, NR4 7TJ, UK, 3Centre for Neuroregeneration Research, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA and 4College of Life Sciences -

The Dorsal Neural Tube Organizes the Dermamyotome and Induces Axial Myocytes in the Avian Embryo
Development 122, 231-241 (1996) 231 Printed in Great Britain © The Company of Biologists Limited 1996 DEV3253 The dorsal neural tube organizes the dermamyotome and induces axial myocytes in the avian embryo Martha S. Spence1, Joseph Yip2 and Carol A. Erickson1,* 1Section of Molecular and Cellular Biology, University of California, Davis, CA 95616, USA 2Department of Neurobiology, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA *Author for correspondence (e-mail: [email protected]) SUMMARY Somites, like all axial structures, display dorsoventral reported previously to induce sclerotome. Thus, we have polarity. The dorsal portion of the somite forms the der- demonstrated that in the context of the embryonic envi- mamyotome, which gives rise to the dermis and axial mus- ronment, a dorsalizing signal from the dorsal neural tube culature, whereas the ventromedial somite disperses to can compete with the diffusible ventralizing signal from the generate the sclerotome, which later comprises the notochord. vertebrae and intervertebral discs. Although the neural In contrast to dorsal neural tube, pieces of ventral neural tube and notochord are known to regulate some aspects of tube, dorsal ectoderm or neural crest cells, all of which this dorsoventral pattern, the precise tissues that initially have been postulated to control dermamyotome formation specify the dermamyotome, and later the myotome from it, or to induce myogenesis, either fail to do so or provoke only have been controversial. Indeed, dorsal and ventral neural minimal inductive responses in any of our assays. However, tube, notochord, ectoderm and neural crest cells have all complicating the issue, we find consistent with previous been proposed to influence dermamyotome formation or to studies that following ablation of the entire neural tube, regulate myocyte differentiation.