Faraday Discussions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-8-2013 12:00 AM Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling) Rana Sabouni The University of Western Ontario Supervisor Prof. Sohrab Rohani The University of Western Ontario Graduate Program in Chemical and Biochemical Engineering A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Rana Sabouni 2013 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Other Chemical Engineering Commons Recommended Citation Sabouni, Rana, "Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)" (2013). Electronic Thesis and Dissertation Repository. 1472. https://ir.lib.uwo.ca/etd/1472 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. i CARBON DIOXIDE ADSORPTION BY METAL ORGANIC FRAMEWORKS (SYNTHESIS, TESTING AND MODELING) (Thesis format: Integrated Article) by Rana Sabouni Graduate Program in Chemical and Biochemical Engineering A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada Rana Sabouni 2013 ABSTRACT It is essential to capture carbon dioxide from flue gas because it is considered one of the main causes of global warming. Several materials and various methods have been reported for the CO2 capturing including adsorption onto zeolites, porous membranes, and absorption in amine solutions. -

New Journal and Database Subscriptions – 2012 -2013
NEW JOURNAL AND DATABASE SUBSCRIPTIONS – 2012 -2013 New Journals Afterall: A Journal of Art, Context and Enquiry American Biology Teacher American Journal of Bioethics American Political Thought Annals of Tourism Research Art Documentation Biodiversity and Conservation Biomaterials Science BioScience Boom: A Journal of California California Archaeology California Management Review Catalysis Science & Technology Chemical Hazards in Industry China Journal Classical Antiquity Classical Philology Crime and Justice Critical Review of International Social and Political Philosophy Education in Chemistry Educational Technology Research Development Elephant Ethics Federal Sentencing Reporter Food & Function Frankie Gastronomica: The Journal of Food and Culture Haaretz Historical Studies in the Natural Sciences HOPOS: The Journal of the International Society for the History of Philosophy of Science Huntington Library Quarterly Indian Country Today Indonesia Journal Information, Communication & Society Innovation Policy and the Economy Integrative Biology Issues in Environmental Science and Technology Journal of Applied Remote Sensing Journal of Digital Media Management Journal of Empirical Research on Human Research Ethics Journal of Environmental Studies and Sciences Journal of Human Capital Journal of Labor Economics Journal of Leisure Research Journal of Micro/Nanolithography, MEMS, and MOEMS Journal of Modern History Journal of Nanophotonics Journal of North African Studies Journal of Palestine Studies Journal of Photonics for Energy Journal -

RSC Gold 2015 Flyer.Pdf
RSC Gold Want access to full content from the world’s leading chemistry society? Including regular new material and an Archive dating back to 1841? Caltech’s RSC Gold Plus voucher codes to publish package subscription has been a very Open Access (OA) free of charge? welcome development ... I am very appreciative of the RSC Gold is the Royal Society of Chemistry’s general excellence of articles in the RSC premium package comprising 41 international research journals, evidenced by strong journals, literature updating services and impact factors and magazines that will meet the needs of all your increases in local download statistics. end-users. And the accompanying Gold for Gold Dana L. Roth OA voucher codes ensure maximum visibility for Chemistry Librarian your institution’s quality research. Caltech, USA Take a look inside to see exactly what you get www.rsc.org/gold RSC Gold includes a wealth of quality RSC journal, database and magazine content that is all available online. Journals Natural Product Reports Analyst New Journal of Chemistry Analytical Methods Organic & Biomolecular Chemistry Biomaterials Science Photochemical & Photobiological Sciences Catalysis Science & Technology Physical Chemistry Chemical Physics (PCCP) Chemical Communications Polymer Chemistry Chemical Science* RSC Advances Chemical Society Reviews Soft Matter CrystEngComm Toxicology Research Dalton Transactions Energy & Environmental Science B a c k fi l e Environmental Science: Nano** RSC Journals Archive 1841-2007 lease Environmental Science: Processes & Impacts -

Publishing Price List 2016
Publishing Price List 2016 Royal Society of Chemistry Collections for 2016 RSC GOLD INCLUDES: Key Royal Society of Chemistry online Price on application ONLINE ONLY† journal, database and magazine content, plus a EMAIL [email protected] book series. or contact your Account Manager PRICES JOURNALS ARCHIVE ONLINE ONLY† RSC Journals Archive Outright Purchase (1841 – 2007) • £41,097 • $72,615 RSC Journals Archive Outright Purchase (2005 – 2007) • £4,867 • $8,271 RSC Journals Archive Lease (1841 – 2007) • £7,408 • $13,133 PRICES RSC Journals Archive Hosting Fee • £819 • $1,340 THE HISTORICAL COLLECTION INCLUDES: Price on application ONLINE ONLY† • Society Publications (1949 – 2012) EMAIL [email protected] • Society Minutes (1841 – 1966) or contact your Account Manager • Historical Papers (1505 – 1991) PRICES CORE CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Dalton Transactions • Journal of Materials Chemistry A, B & C • New Journal of Chemistry PRINT & ONLINE† ONLINE ONLY† • Organic & Biomolecular Chemistry • • • Physical Chemistry Chemical Physics £19,685 £18,701 • RSC Advances (online only) • $36,814 • $34,973 PRICES GENERAL CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Chemical Society Reviews PRINT & ONLINE† ONLINE ONLY† • Chemistry World • £8,360 • £7,942 • New Journal of Chemistry PRICES • $13,685 • $13,244 • RSC Advances (online only) ANALYTICAL SCIENCE COLLECTION INCLUDES: • Analyst • Analytical Abstracts (online only) • Analytical Methods PRINT & ONLINE† ONLINE ONLY† • Environmental Science: Processes & Impacts • -

Annual Review 2011
Annual Review 2011 www.rsc.org Contents 01 Welcome from the President 02 A message from the Chief Executive 03 Supporting a strong membership 07 Leading the global chemistry community 11 Engaging people with chemistry 15 Influencing the future of chemistry 19 Enhancing knowledge 23 Summary of financial information 24 Contacts Professor David Phillips CBE CSci CChem FRSC We championed the cause of chemical sciences with pride and conviction throughout the International Year of Chemistry. ‘‘ Welcome from the President When the United Nations announced that 2011 would be designated the “International Year of Chemistry” (IYC), we knew immediately that the year would bring countless opportunities to promote, expand and evolve both the RSC and the chemical sciences more broadly. We needed to make it a year to remember. I’m delighted to say we rose to the challenge. In a year marked with natural disasters, economic uncertainty and adverse conditions affecting the chemical sciences in ways never seen before, we still led the UK in being perhaps the most active country in the world throughout IYC. Our members did us proud, arranging hundreds of IYC events across the globe. Perhaps most visible was the Global Water Experiment, an international effort to map global water quality using data collected by school pupils. A national media campaign, including an outing on BBC TV’s One Show, led to widespread awareness of the experiment. Dedicated and enthusiastic UK teachers then inspired a sensational number of their pupils to take part, and as a country we contributed more data to the experiment than any other. -

GUT RSC Journals List
SCHEDULE A Publisher Content Section A Customer has access to the electronic versions of the following journals via an External route: Access Post - Copyright Journals E-ISSN years cancellation Owner* during Term access Analyst 1364-5528 2008-2018 2012-2018 RSC Analytical Methods 1 1759-9679 2009-2018 2012-2018 RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2008-2013 2012-2013 RSC B 1460 4779 2008-2013 2012-2013 RSC C 1460-4787 2008-2013 2012-2013 RSC Biomaterials Science 1 2047-4849 2013-2018 2016-2018 RSC Catalysis Science & Technology 1 2044-4761 2011-2018 2013-2018 RSC Chemical Communications 1364-548X 2008-2018 2012-2018 RSC Chemical Science 1, 2 2041-6539 2010-2014 2012-2014 RSC Chemical Society Reviews 1460-4744 2008-2018 2012-2018 RSC Chemistry World 1749-5318 2012-2016 2012-2016 RSC CrystEngComm 1466-8033 2008-2018 2012-2018 RSC Dalton Transactions 1477-9234 2008-2018 2012-2018 RSC Education in Chemistry 1749-5326 2012-2016 2012-2016 RSC Energy & Environmental Science 1 1754-5706 2008-2018 2012-2018 RSC Environmental Science: Nano 1 2051-8161 2014-2018 2016-2018 RSC Environmental Science: Processes & Impacts including 2050-7895 2013-2018 2013-2018 RSC Journal of Environmental Monitoring (1464-0333) 2008-2012 2012 Environmental Science: Water Research & Technology 1 2053-1419 2015-2018 2017-2018 RSC Faraday Discussions 1364-5498 2008-2018 2012-2018 RSC Food & Function 1 2042-650X 2010-2018 2012-2018 RSC Green Chemistry 1463-9270 2008-2018 2012-2018 RSC Inorganic Chemistry Frontiers 1 2052-1553 2014-2018 2017-2018 -
SCHEDULE B Publisher Content
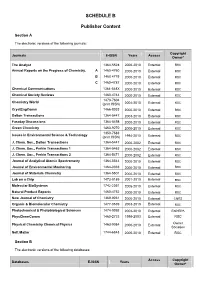
SCHEDULE B Publisher Content Section A The electronic versions of the following journals: Copyright Journals E-ISSN Years Access Owner* The Analyst 1364-5528 2000-2010 External RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2000-2010 External RSC B 1460 4779 2000-2010 External RSC C 1460-4787 2000-2010 External RSC Chemical Communications 1364-548X 2000-2010 External RSC Chemical Society Reviews 1460-4744 2000-2010 External RSC 1473-7604 Chemistry World (print ISSN) 2004-2010 External RSC CrystEngComm 1466-8033 2000-2010 External RSC Dalton Transactions 1364-5447 2003-2010 External RSC Faraday Discussions 1364-5498 2000-2010 External RSC Green Chemistry 1463-9270 2000-2010 External RSC 1350-7583 Issues in Environmental Science & Technology (print ISSN) 1994-2010 External RSC J. Chem. Soc., Dalton Transactions 1364-5447 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 1 1364-5463 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 2 1364-5471 2000-2002 External RSC Journal of Analytical Atomic Spectrometry 1364-5544 2000-2010 External RSC Journal of Environmental Monitoring 1464-0333 2000-2010 External RSC Journal of Materials Chemistry 1364-5501 2000-2010 External RSC Lab on a Chip 1473-0189 2001-2010 External RSC Molecular BioSystems 1742-2051 2005-2010 External RSC Natural Product Reports 1460-4752 2000-2010 External RSC New Journal of Chemistry 1369-9261 2000-2010 External CNRS Organic & Biomolecular Chemistry 1477-0539 2003-2010 External RSC Photochemical & Photobiological Sciences 1474-9092 2002-2010 -

Alexander Ruf Dissertation
TECHNISCHE UNIVERSITÄT MÜNCHEN Previously unknown organomagnesium compounds in astrochemical context Alexander Ruf Dissertation TECHNISCHE UNIVERSITÄT MÜNCHEN Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt Lehrstuhl für Analytische Lebensmittelchemie Previously unknown organomagnesium compounds in astrochemical context Alexander Ruf Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigten Dissertation. Vorsitzender: Prof. Dr. Erwin Grill Prüfer der Dissertation: 1. apl. Prof. Dr. Philippe Schmitt-Kopplin 2. Prof. Dr. Michael Rychlik 3. Prof. Eric Quirico, PhD (Université Grenoble Alpes) Die Dissertation wurde am 06.12.2017 bei der Technischen Universität München ein- gereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 18.01.2018 angenommen. Do we feel less open-minded, the more open-minded we are? A tribute to sensitivity and resolution... Acknowledgments This work has been prepared at the Helmholtz Zentrum München in the research unit Analytical BioGeoChemistry of apl. Prof. Dr. Philippe Schmitt-Kopplin, in collaboration with the Chair of Analytical Food Chemistry at the Technical Uni- versity of Munich. In the course of these years, I have relied on the courtesy and support of many to which I am grateful. The success of this PhD thesis would not have been possible without help and support of many wonderful people. First of all, I would like to thank the whole research group Analytical BioGeo- Chemistry for a very friendly, informal, and emancipated working atmosphere that formed day-by-day an enjoyable period of residence - it has felt like freedom! Small issues like having stimulating lunch discussions or going out into a bar, friendly peo- ple could be found herein to setting up a balance to scientific work. -

Products by Subject 2019 Your Reference Guide
For more information, please Products by subject 2019 contact your account manager Your reference guide or email [email protected] *2018 Journal Citation Reports based on 2017 Web of Science data. © Clarivate Analytics, 2018 © Clarivate Science data. of Reports based on 2017 Web *2018 Journal Citation Journals **Impact factor pending. IMPACT FACTOR* IMPACT Analytical Biological Catalysis Chemical Biology & Medicinal Energy Environmental Food General Chemistry Inorganic Materials Nanoscience Organic Physical Analyst 3.864 Analytical Methods 2.073 Biomaterials Science 5.831 Catalysis Science & Technology 5.365 ChemComm 6.290 Chemical Science 9.063 Chem Soc Rev 40.182 Chemistry Education Research and Practice 1.621 CrystEngComm 3.304 Dalton Transactions 4.099 Energy & Environmental Science 30.067 Environmental Science: Nano 6.087 Environmental Science: Processes & Impacts 2.491 Environmental Science: 3.649 Water Research & Technology Faraday Discussions 3.427 Food & Function 3.289 Green Chemistry 8.586 Inorganic Chemistry Frontiers 5.106 JAAS (Journal of Analytical Atomic Spectrometry) 3.608 Journals of Materials Chemistry A 9.931 Journals of Materials Chemistry B 4.776 Journals of Materials Chemistry C 5.976 Lab on a Chip 5.995 Materials Chemistry Frontiers ** Materials Horizons 13.183 MedChemComm 2.342 Metallomics 4.069 Molecular Omics ** MSDE (Molecular Systems Design & Engineering) ** Nanoscale 7.233 Nanoscale Advances ** Nanoscale Horizons 9.391 Natural Product Reports (NPR) 11.406 New Journal of Chemistry (NJC) 3.201 Organic & Biomolecular -

CONFERENCE REPORT View Journal | View Issue
ChemComm View Article Online CONFERENCE REPORT View Journal | View Issue Highlights from Faraday Discussion FDSERS17: Surface Enhanced Raman Scattering – SERS, Cite this: Chem. Commun., 2017, 53, 12726 Glasgow, UK, 30th August–1st September 2017 G. Di Martino, †a H. Fleming,†b M. Kamp †*c and F. Lussier†d The 2017 Faraday Discussion on Surface Enhanced Raman Scattering (SERS) attracted more than a hundred delegates from a broad spectrum of backgrounds and experience levels, bringing together DOI: 10.1039/c7cc90411h leading scientists involved in the long living field of SERS. The meeting gave an overview of the liveliness of the topic, characterised by open questions and fascinating science still to discover. In the following, rsc.li/chemcomm we discuss the topics covered during this meeting and briefly highlight the content of each presentation. On 30th August 2017, around 140 delegates with the format of Faraday Discussions, Opening lecture representing 30 different countries all of the speakers submitted a paper gathered in a remarkably sunny Glasgow, prior to this meeting, which was made With his 45 minute opening lecture, at the University of Strathclyde, to attend available for all of the participants to read Prof. R. Van Duyne set the scene for the the 2017 Faraday Discussion on Surface before the event. During their respective discussion, providing a stimulating and Enhanced Raman Scattering (Fig. 1). sessions, the presenters were given wide-ranging introduction to the field of ‘‘The discussion meeting brought together 5 minutes each to briefly summarize SERS. Prof. Van Duyne noted that the a real mix of SERS researchers embracing the main findings of their work, followed previous Faraday Discussions on SERS chemistry, physics, and engineering. -

Electronic Access Licence Agreement
ELECTRONIC ACCESS LICENCE AGREEMENT This Agreement is made the Chemistry located at ("Publisher"} and Th offices at WHEREAS (A) Publisher holds journal articles and databases in electronic form; (B) Customer wishes to license access to journal articles and/or databases; and (C) Publisher agrees to grant such licence. NOW, THEREFORE, in consideration of the mutual promises set forth herein, the parties agree as follows: 1. Definitions In thisAgreement the following terms shall have the following meanings: "Authorised Users" means current employees (including faculty, staff, and independent contractors) of the Customer and students ofthe Customer who, in either case, have been allowed access to Publlsher Content by Customer, regardless of the physical location of such persons, such access shall be unlimited and shall be by Secure Authentication so that these users may access and use Publisher Content in accordance with the terms of this Agreement. Remote access by AuthorisedUsers to Publisher Content is allowed and Walk-In Users, i.e. persons who are permitted by the Customer to access Publisher Content whilst they are on Customer's physical premises. Such access must at all times be by Secure Authentication on computer terminals within the Customer's physical premises. Walk-In Users may not be given means to access Publisher Content outside the Customer's physical premises or by any wireless network unless the network is a secure network provided by the Customer. "Commercial Use" means use which is for direct monetary reward or commercial advantage, whether by or for Customer or Authorised User, by means of the sale, resale, loan, transfer, hire or other form of exploitation of Publisher Content. -
Products by Subject 2019 Your Reference Guide
For more information, please Products by subject 2019 contact your account manager Your reference guide or email [email protected] *2018 Journal Citation Reports based on 2017 Web of Science data. © Clarivate Analytics, 2018 © Clarivate Science data. of Reports based on 2017 Web *2018 Journal Citation Journals **Impact factor pending. IMPACT FACTOR* IMPACT Analytical Biological Catalysis Chemical Biology & Medicinal Energy Environmental Food General Chemistry Inorganic Materials Nanoscience Organic Physical Analyst 3.864 Analytical Methods 2.073 Biomaterials Science 5.831 Catalysis Science & Technology 5.365 ChemComm 6.290 Chemical Science 9.063 Chem Soc Rev 40.182 Chemistry Education Research and Practice 1.621 CrystEngComm 3.304 Dalton Transactions 4.099 Energy & Environmental Science 30.067 Environmental Science: Nano 6.087 Environmental Science: Processes & Impacts 2.491 Environmental Science: 3.649 Water Research & Technology Faraday Discussions 3.427 Food & Function 3.289 Green Chemistry 8.586 Inorganic Chemistry Frontiers 5.106 JAAS (Journal of Analytical Atomic Spectrometry) 3.608 Journals of Materials Chemistry A 9.931 Journals of Materials Chemistry B 4.776 Journals of Materials Chemistry C 5.976 Lab on a Chip 5.995 Materials Chemistry Frontiers ** Materials Horizons 13.183 MedChemComm 2.342 Metallomics 4.069 Molecular Omics ** MSDE (Molecular Systems Design & Engineering) ** Nanoscale 7.233 Nanoscale Advances ** Nanoscale Horizons 9.391 Natural Product Reports (NPR) 11.406 New Journal of Chemistry (NJC) 3.201 Organic & Biomolecular