Determine Grounds for a Reassessment of an Existing New Organisms Approval
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Custom Trip
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Custom Trip October 20—November 6, 2016 Guide: Ken Behrens All photos taken during this trip by Ken Behrens Annotated bird list by Jerry Connolly TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with the opening of a satellite office in the country several years ago, we further solidified our expertise in the “Eighth Continent.” This custom trip followed an itinerary similar to that of our main set-departure tour. Although this trip had a definite bird bias, it was really a general natural history tour. We took our time in observing and photographing whatever we could find, from lemurs to chameleons to bizarre invertebrates. Madagascar is rich in wonderful birds, and we enjoyed these to the fullest. But its mammals, reptiles, amphibians, and insects are just as wondrous and accessible, and a trip that ignored them would be sorely missing out. We also took time to enjoy the cultural riches of Madagascar, the small villages full of smiling children, the zebu carts which seem straight out of the Middle Ages, and the ingeniously engineered rice paddies. If you want to come to Madagascar and see it all… come with Tropical Birding! Madagascar is well known to pose some logistical challenges, especially in the form of the national airline Air Madagascar, but we enjoyed perfectly smooth sailing on this tour. We stayed in the most comfortable hotels available at each stop on the itinerary, including some that have just recently opened, and savored some remarkably good food, which many people rank as the best Madagascar Custom Tour October 20-November 6, 2016 they have ever had on any birding tour. -

Universidad De El Salvador Facultad Mutidisciplinaria De Occidente Departamento De Biología
UNIVERSIDAD DE EL SALVADOR FACULTAD MUTIDISCIPLINARIA DE OCCIDENTE DEPARTAMENTO DE BIOLOGÍA INVENTARIO PRELIMINAR DE MARIPOSAS DIURNAS EN EL AREA NATURAL PROTEGIDA LA MAGDALENA, MUNICIPIO DE CHALCHUAPA, DEPARTAMENTO DE SANTA ANA, DURANTE EL PERIODO COMPRENDIDO ENTRE NOVIEMBRE 2012 A JULIO 2013 PRESENTADO POR LOVOS DE ARÉVALO, ALEXIS YANILET REYES DE GÓCHEZ, CLAUDIA CAROLINA PARA OPTAR AL GRADO DE LICENCIADAS EN BIOLOGÍA DOCENTES DIRECTORES LICENCIADO CARLOS MAURICIO LINARES HERNÁNDEZ ING. AGR. JOSÉ MIGUEL SERMEÑO CHICAS COORDINADOR DE TRABAJOS DE GRADO LICENCIADO OSCAR ARMANDO GUERRA JUNIO, 2014 SANTA ANA EL SALVADOR CENTROAMÉRICA UNIVERSIDAD DE EL SALVADOR FACULTAD MULTIDISCIPLINARIA DE OCCIDENTE DEPARTAMENTO DE BIOLOGÍA INVENTARIO PRELIMINAR DE MARIPOSAS DIURNAS EN EL AREA NATURAL PROTEGIDA LA MAGDALENA, MUNICIPIO DE CHALCHUAPA, DEPARTAMENTO DE SANTA ANA, DURANTE EL PERIODO COMPRENDIDO ENTRE NOVIEMBRE 2012 A JULIO 2013 PRESENTADO POR LOVOS DE ARÉVALO, ALEXIS YANILET REYES DE GÓCHEZ, CLAUDIA CAROLINA PARA OPTAR AL GRADO DE LICENCIADAS EN BIOLOGÍA DOCENTES DIRECTORES LICENCIADO CARLOS MAURICIO LINARES HERNÁNDEZ ING. AGR. JOSÉ MIGUEL SERMEÑO CHICAS COORDINADOR GENERAL DE PROCESOS DE GRADO LIC. OSCAR ARMANDO GUERRA ASENCIO JUNIO, 2014 SANTA ANA EL SALVADOR CENTROAMÉRICA UNIVERSIDAD DE EL SALVADOR FACULTAD MUTIDISCIPLINARIA DE OCCIDENTE DEPARTAMENTO DE BIOLOGÍA INVENTARIO PRELIMINAR DE MARIPOSAS DIURNAS EN EL AREA NATURAL PROTEGIDA LA MAGDALENA, MUNICIPIO DE CHALCHUAPA, DEPARTAMENTO DE SANTA ANA, DURANTE EL PERIODO COMPRENDIDO ENTRE NOVIEMBRE 2012 A JULIO 2013 PRESENTADO POR LOVOS DE ARÉVALO, ALEXIS YANILET REYES DE GÓCHEZ, CLAUDIA CAROLINA PARA OPTAR AL GRADO DE LICENCIADAS EN BIOLOGÍA DOCENTE DIRECTOR: LIC. CARLOS MAURICIO LINARES HERNÁNDEZ________________ (Firma) COORDINADOR GENERAL DE PROCESOS DE GRADO LIC. OSCAR ARMANDO GUERRA ASENCIO____________________ (Firma) JUNIO, 2014 SANTA ANA EL SALVADOR CENTROAMÈRICA UNIVERSIDAD DE EL SALVADOR RECTOR ING. -

(Bakalářská Práce, Maurer, Bozo-Ks 2014
JIHO ČESKÁ UNIVERZITA V ČESKÝCH BUD ĚJOVICÍCH ZEM ĚDĚLSKÁ FAKULTA Studijní program: B4106 Zem ědělská specializace Studijní obor: Biologie a ochrana zájmových organism ů Katedra: Katedra biologických disciplín Vedoucí katedry: doc. RNDr. Ing. Josef Rajchard, Ph.D. Bakalá řská práce Obchod s motýly pod ochranou CITES Vedoucí bakalá řské práce: RNDr. Josef Navrátil, Ph.D. Autor bakalá řské práce: Jan Maurer České Bud ějovice 2014 Prohlašuji, že v souladu s § 47b zákona č. 111/1998 Sb. v platném zn ění souhlasím se zve řejn ěním své bakalá řské práce, a to – v nezkrácené podob ě – v úprav ě vzniklé vypušt ěním vyzna čených částí archivovaných Zem ědělskou fakultou – elektronickou cestou ve ve řejn ě p řístupné části databáze STAG provozované Jiho českou univerzitou v Českých Bud ějovicích na jejích internetových stránkách, a to se zachováním mého autorského práva k odevzdanému textu této kvalifika ční práce. Souhlasím dále s tím, aby toutéž elektronickou cestou byly v souladu s uvedeným ustanovením zákona č. 111/1998 Sb. zve řejn ěny posudky školitele a oponent ů práce i záznam o pr ůběhu a výsledku obhajoby kvalifika ční práce. Rovn ěž souhlasím s porovnáním textu mé kvalifika ční práce s databází kvalifika čních prací Theses.cz provozovanou Národním registrem vysokoškolských kvalifika čních prací a systémem na odhalování plagiát ů. Datum 11. 4. 2014 Děkuji p ředevším mému školiteli RNDr. Josefu Navrátilovi, Ph.D. za vedení bakalá řské práce a paní doc. RNDr. Iv ě Dostálkové, Ph.D. za pomoc se zpracováním graf ů a statistiky. Taktéž d ěkuji všem ostatním, kte ří mi poskytli užite čné rady pro vypracování této práce. -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

P. 1 AC17 Doc. 7.2 CONVENTION on INTERNATIONAL TRADE in ENDANGERED SPECIES of WILD FAUNA and FLORA
AC17 Doc. 7.2 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Seventeenth meeting of the Animals Committee Hanoi (Viet Nam), 30 July-3 August 2001 Implementation of Resolution Conf. 8.9 (Rev.) REVIEW OF THE IMPLEMENTATION OF RECOMMENDATIONS (PART I: LIST OF SPECIES PREVIOUSLY REVIEWED) This document has been prepared by the Secretariat. 1. At the 16th meeting of the Animals Committee, the Secretariat informed the Committee that it had decided to review the implementation of all recommendations that have been formulated by the Committee in the context of Resolution Conf. 8.9 (Rev.) and that the Committee would be informed of the results of this review, which was expected to become available over the following 12 to 18 months. The Committee supported this initiative. 2. The Secretariat was requested to provide a list of animal species that were or had been subject to the Review of Significant Trade for the 17th meeting of the Animals Committee. 3. The Annex to this document presents, as a first step, a list of all the animal species that have been reviewed pursuant to Resolution Conf. 8.9 (Rev.) and for which the Committee has formulated recommendations. This Annex is the result of Part I of the review referred to in paragraph 1. Part II, which will describe the implementation of earlier primary and secondary recommendations made by the Animals Committee concerning species that were included in the Review of Significant Trade, will be presented at a future meeting of the Committee. AC17 Doc. 7.2 – p. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Flexural Stiffness Patterns of Butterfly Wings (Papilionoidea)
35:61–77,Journal of Research1996 (2000) on the Lepidoptera 35:61–77, 1996 (2000) 61 Flexural stiffness patterns of butterfly wings (Papilionoidea) Scott J. Steppan Committee on Evolutionary Biology, University of Chicago, Chicago, IL 60637, USA., E-mail: [email protected] Abstract. A flying insect generates aerodynamic forces through the ac- tive manipulation of the wing and the “passive” properties of deformability and wing shape. To investigate these “passive” properties, the flexural stiffness of dried forewings belonging to 10 butterfly species was compared to the butterflies’ gross morphological parameters to determine allom- etric relationships. The results show that flexural stiffness scales with wing 3.9 loading to nearly the fourth power (pw ) and is highly correlated with wing area cubed (S3.1). The generalized map of flexural stiffness along the wing span for Vanessa cardui has a reduction in stiffness near the distal tip and a large reduction near the base. The distal regions of the wings are stiffer against forces applied to the ventral side, while the basal region is much stiffer against forces applied dorsally. The null hypothesis of structural isom- etry as the explanation for flexural stiffness scaling is rejected. Instead, selection for a consistent dynamic wing geometry (angular deflection) in flight may be a major factor controlling general wing stiffness and deformability. Possible relationships to aerodynamic and flight habit fac- tors are discussed. This study proposes a new approach to addressing the mechanics of insect flight and these preliminary results need to be tested using fresh wings and more thorough sampling. KEY WORDS: biomechanics, butterfly wings, flight, allometry, flexural stiff- ness, aerodynamics INTRODUCTION A flying insect generates aerodynamic forces primarily through the ac- tive manipulation of wing movements and the “passive” morphological prop- erties of deformability and wing shape. -

1 Annual Portfolio Overview Wallacea Biodiversity Hotspot 30 June 2018
Annual Portfolio Overview Wallacea Biodiversity Hotspot 30 June 2018 (FY 18) 1. Introduction The Wallacea region, which includes the whole of Timor-Leste and the central portion of Indonesia, including the major island groups of Sulawesi, Maluku, and the Lesser Sundas, qualifies as a hotspot due to its high levels of plant endemism and extensive habitat loss. The chief causes of habitat loss include overexploitation of natural resources, degradation, fragmentation, and conversion, and pressure from human population growth and economic development. Wallacea is an island landscape, with over 1,680 islands and 30 million people, the majority of whom live in coastal areas earning their living from farms, forests, wetlands, and the sea. The Wallacea region, first described biologically by Alfred Russel Wallace in 1869, is noteworthy for having fauna and flora that are distinct from the Asian biogeographic realm to the west and the Australian-Pacific biogeographic realm to the south and east. The many islands are varied – volcanic, non-volcanic, continental crusts, and composites – and are separated by shallow seas in some cases and trenches as deep as 7,000 meters in others. Powerful currents connecting the Pacific and Indian Oceans flow through the region, creating barriers to dispersal of species. The complex geography and barriers to movement have led to the region’s high biodiversity. Among the hotspot’s endemic species are 1,500 vascular plants, 127 mammals, 274 birds, 99 reptiles, 33 amphibians, 50 freshwater fish, and 110 marine fish. There are also as many as 400 species of coral in the region. Notable endemic species include tarsiers, macaques, Flores hawk-eagle (Nisaetus floris), and Komodo dragon (Varanus komodoensis). -

Papilionoidea De La Sierra De Huautla, Morelos Y Puebla, México (Insecta: Lepidoptera)
Papilionoidea de la Sierra de Huautla, Morelos y Puebla, México (Insecta: Lepidoptera) Mercedes Luna-Reyes1, Jorge Llorente-Bousquets2* & Armando Luis-Martínez2 1. Museo de Zoología, Facultad de Estudios Superiores Zaragoza, Av. Guelatao No. 66. Col. Ejército de Oriente. Iztapalapa. México D.F. 09230; [email protected], [email protected] 2. Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510. *Responsable de la publicación; jlb@ hp.fciencias.unam.mx Recibido 04-VIII-2007. Corregido 30-VI-2008. Aceptado 31-VII-2008. Abstract: Papilionoidea from Sierra de Huautla, Morelos and Puebla, México (Insecta: Lepidoptera). The Cuenca del Balsas region has significant biodiversity and endemicity of its herpetofauna, avifauna and vascular plants. Despite this, our knowledge of the Papilionoidea of the region is poor. We analyzed the local and temporal distribution of Papilionoidea at 24 localities in the states of Morelos and Puebla. The study sites are situated between 900 and 1300 m. a. s. l., and are composed of dry tropical forest (dtf). We recorded 8790 individuals of 83 genera and 142 species of Papilionoidea (sensu Kristensen, 1975), over 79 days of field work, with 2-4 days at each of the 24 localities. Twenty five species were newly recorded for the state of Puebla. Our data render Morelos and Puebla among the seven richest Mexican states, in terms of Papilionoidea diversity. Our results show that the Sierra de Huautla has the lowest diversity, but the highest standard abundance, compared to other Mexican regions with similar vegetation. Patterns of diversity and seasonal abundance are atypical, in that individuals of many species are unusually abundant during the wet months. -
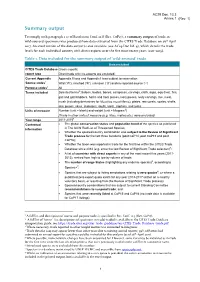
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Madagascar 2017
Field Guides Tour Report Madagascar 2017 Nov 6, 2017 to Nov 27, 2017 Phil Gregory & Doug Gochfeld For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. We started the tour in the virtually pristine rainforests of Ranomafana, which the group (along with Gerard at right and Baku at far left) is enjoying here just prior to our dusk search for mouse lemurs. Photo by guide Doug Gochfeld. This year’s Field Guides Madagascar tour was full of great features, including over twenty species of Lemur, more than one hundred species of endemic birds, and a litany of fascinating natural wonders and cultural insights. Indeed, there were so many highlights that there wasn’t widespread agreement about just what the favorite bird or mammal or experience of the trip was. We fared very well with the weather; we had some cool and cloudy conditions to relieve us from the unforgiving tropical sun in a couple of locations, and the periods of rain mostly kept away from our birding excursions. Other than the plague scare that wasn’t so scary after all, the bees of Andasibe, and a very minor Mad Air schedule change, the tour went off without so much as a hitch! This year, we started out by heading to Ranomafana National Park, one of the real jewels among Madagascar’s protected areas. Having this as our first stop means two days of mostly driving to start the tour, but to everyone’s credit, it was handled gracefully and without complaint. We managed to fit in a couple of very productive birding stops on the way south: on day one at a nice flooded rice paddy area, where we scored our only Madagascar Snipes of the tour along with a swirling mass of Plain (Brown-throated) Martins, and then on day two at the Reserve Villageoise D’Ankazomivady, where the highlight among introductions to several Madagascar endemic species was a scarce Baillon’s Crake! We spent an afternoon and then two full days exploring the rainforests around Ranomafana, including the “Circuit 2” trail on the way to Vohiparara.