Marine Protected Area Design and the Spatial and Temporal Distribution of Cetaceans in a Submarine Canyon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characteristics and Importance of Rill and Gully Erosion: a Case Study in a Small Catchment of a Marginal Olive Grove
Cuadernos de Investigación Geográfica 2015 Nº 41 (1) pp. 107-126 ISSN 0211-6820 DOI: 10.18172/cig.2644 © Universidad de La Rioja CHARACTERISTICS AND IMPORTANCE OF RILL AND GULLY EROSION: A CASE STUDY IN A SMALL CATCHMENT OF A MARGINAL OLIVE GROVE E.V. TAGUAS1*, E. GUZMÁN1, G. GUZMÁN1, T. VANWALLEGHEM1, J.A. GÓMEZ2 1Rural Engineering Department/ Agronomy Department, University of Cordoba, Campus Rabanales, Leonardo Da Vinci building, 14071 Córdoba, Spain. 2Institute for Sustainable Agriculture, CSIC, Apartado 4084, 14080 Córdoba, Spain. ABSTRACT. Measurements of gullies and rills were carried out in an olive or- chard microcatchment of 6.1 ha over a 4-year period (2010-2013). No tillage management allowing the development of a spontaneous grass cover was imple- mented in the study period. Rainfall, runoff and sediment load were measured at the catchment outlet. The objectives of this study were: 1) to quantify erosion by concentrated flow in the catchment by analysis of the geometric and geomor- phologic changes of the gullies and rills between July 2010 and July 2013; 2) to evaluate the relative percentage of erosion derived from concentrated runoff to total sediment yield; 3) to explain the dynamics of gully and rill formation based on the hydrological patterns observed during the study period; and 4) to improve the management strategies in the olive grove. Control sections in gullies were established in order to get periodic measurements of width, depth and shape in each campaign. This allowed volume changes in the concentrated flow network to be evaluated over 3 periods (period 1 = 2010-2011; period 2 = 2011-2012; and period 3 = 2012-2013). -

Alluvial Fans in the Death Valley Region California and Nevada
Alluvial Fans in the Death Valley Region California and Nevada GEOLOGICAL SURVEY PROFESSIONAL PAPER 466 Alluvial Fans in the Death Valley Region California and Nevada By CHARLES S. DENNY GEOLOGICAL SURVEY PROFESSIONAL PAPER 466 A survey and interpretation of some aspects of desert geomorphology UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1965 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publications as follows: Denny, Charles Storrow, 1911- Alluvial fans in the Death Valley region, California and Nevada. Washington, U.S. Govt. Print. Off., 1964. iv, 61 p. illus., maps (5 fold. col. in pocket) diagrs., profiles, tables. 30 cm. (U.S. Geological Survey. Professional Paper 466) Bibliography: p. 59. 1. Physical geography California Death Valley region. 2. Physi cal geography Nevada Death Valley region. 3. Sedimentation and deposition. 4. Alluvium. I. Title. II. Title: Death Valley region. (Series) For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C., 20402 CONTENTS Page Page Abstract.. _ ________________ 1 Shadow Mountain fan Continued Introduction. ______________ 2 Origin of the Shadow Mountain fan. 21 Method of study________ 2 Fan east of Alkali Flat- ___-__---.__-_- 25 Definitions and symbols. 6 Fans surrounding hills near Devils Hole_ 25 Geography _________________ 6 Bat Mountain fan___-____-___--___-__ 25 Shadow Mountain fan..______ 7 Fans east of Greenwater Range___ ______ 30 Geology.______________ 9 Fans in Greenwater Valley..-----_____. 32 Death Valley fans.__________--___-__- 32 Geomorpholo gy ______ 9 Characteristics of fans.._______-___-__- 38 Modern washes____. -

SCI Lecture Paper Series
SCI LECTURE PAPERS SERIES HIGHWAY DRAINAGE SYSTEMS Santi V Santhalingam Highways Agency, Room 4/41, St. Christopher House Southwark Street, London SE1 0TE Telephone +44 (0) 171 921 4954 Fax +44 (0) 171 921 4411 © Highways Agency 1999 copyright reserved ISSN 1353-114X LPS 102/99 Key words highways, drainage, runoff, surface, sub-surface, systems INTRODUCTION Appropriate drainage is an important feature of good highway design in terms of ensuring required level of service and value for money are achieved. Highway drainage has two major objectives: safety of the road user and longevity of the pavement. Speedy removal of surface water will help to ensure safe and comfortable conditions for the road user. Provision of effective sub-drainage will maximise longevity of the pavement and its associated earthworks. Highway drainage can therefore be broadly classified into two elements – surface run-off and sub-surface run-off: these two elements are not completely disparate in that some of the surface water may find its way into the road foundation through surfaces which are not completely impermeable thence requiring removal by sub-drainage. Based on these fundamental principles, drainage methods in the UK are broadly divided into two categories: (a) combined systems, where the surface and sub-surface water are collected and transported in the same pipe, and (b) separate systems, where the two elements are collected and transported in separate pipes Within the broader definition of the two systems there are a number of different drainage methods that are in use on UK highways, some of them more common than others. -

Marine Protected Areas (Mpas) in Management 1 of Coral Reefs
ISRS BRIEFING PAPER 1 MARINE PROTECTED AREAS (MPAS) IN MANAGEMENT 1 OF CORAL REEFS SYNOPSIS Marine protected areas (MPAs) may stop all extractive uses, protect particular species or locally prohibit specific kinds of fishing. These areas may be established for reasons of conservation, tourism or fisheries management. This briefing paper discusses the potential uses of MPAs, factors that have affected their success and the conditions under which they are likely to be effective. ¾ MPAs are often established as a conservation tool, allowing protection of species sensitive to fishing and thus preserving intact ecosystems, their processes and biodiversity and ultimately their resilience to perturbations. ¾ Increases in charismatic species such as large groupers in MPAs combined with the perception that the reefs there are relatively pristine mean that MPAs can play a significant role in tourism. ¾ By reducing fishing mortality, effective MPAs have positive effects locally on abundances, biomass, sizes and reproductive outputs of many exploitable site- attached reef species. ¾ Because high biomass of focal species is sought but this is quickly depleted and is slow to recover, poaching is a problem in most reef MPAs. ¾ Target-species ‘spillover’ into fishing areas is likely occurring close to the MPA boundaries and benefits will often be related to MPA size. Evidence for MPAs acting as a source of larval export remains weak. ¾ The science of MPAs is at an early stage of its development and MPAs will rarely suffice alone to address the main objectives of fisheries management; concomitant control of effort and other measures are needed to reduce fishery impacts, sustain yields or help stocks to recover. -

Critical Habitats and Biodiversity: Inventory, Thresholds and Governance
Commissioned by BLUE PAPER Summary for Decision-Makers Critical Habitats and Biodiversity: Inventory, Thresholds and Governance Global biodiversity loss results from decades of unsustainable use of the marine environment and rep- resents a major threat to the ecosystem services on which we, and future generations, depend. In the past century, overexploitation of fisheries and the effects of bycatch have caused species to decline whilst coastal reclamation and land-use change—together with pollution and, increasingly, climate change—have led to vast losses of many valuable coastal habitats, estimated at an average of 30–50 percent.1 Despite advances in understanding how marine species and habitats are distributed in the ocean, trends in marine biodiversity are difficult to ascertain. This results from the very patchy state of knowl- edge of marine biodiversity, which leads to biases in understanding different geographic areas, groups of species, habitat distribution and patterns of decline. To address the data gap in our understanding of marine biodiversity and ecosystem integrity, it is crucial to establish the capacity to assess current baselines and trends through survey and monitoring activities. Only increased knowledge and understanding of the above will be able to inform ocean manage- ment and conservation strategies capable of reversing the current loss trend in marine biodiversity. A new paper2 commissioned by the High Level Panel for a Sustainable Ocean Economy increases our understanding of these trends by analysing the links between biodiversity and ecosystem functioning (including tipping points for degradation) and unpacks the link between protection and gross domes- tic product (GDP). In doing so, the paper provides an updated catalogue of marine habitats and biodiversity and outlines five priorities for changing the current trajectory of decline. -

Marine Protected Areas
Marine Protected Areas Read the passage below. How do oceans affect you? If you live far from the coast, you might think they don’t. But life on this planet depends on the oceans. They cover almost three-quarters of the planet and hold 97 percent of the Earth’s water. The phytoplankton that live on the oceans’ surface produce 70 percent of the oxygen in the atmosphere. Oceans are a vital source of food and other resources, and an economic engine for many communities. For all the oceans provide us, we haven’t always been so responsible in our stewardship. “The ocean was thought of as a dumping ground for so long,” says Caitlyn Toropova of the International Union for Conservation of Nature (IUCN). “There was a sense that there was no way we could harm it because it is so vast.” But human activities are having a negative impact on many of the world’s oceans, jeopardizing marine life, habitat, and ecosystems. These threats include overfishing or destructive fishing, coastal development, pollution and runoff, and the introduction of non-native species. Climate change is also having a big effect by causing warming seas and rising acidification. The realization that something needs to be done to stem or reverse the damage has led to the creation of Marine Protected Areas (MPAs). Broadly speaking, marine protected areas are regions of the ocean where human activity is limited. Specifics differ around the globe, but the United States defines a marine protected area as “any area of the marine environment that has been reserved by federal, state, tribal, territorial, or local laws or regulations to provide lasting protection for part or all of the natural and cultural resources therein.” There are approximately 5,000 designated MPAs around the world, and many more that are not officially recognized, says Toropova, the conservation group’s coordination officer for marine protected areas. -

Multi-Zone Marine Protected Areas: Assessment of Ecosystem and Fisheries Benefits Using Multiple Ecosystem Models X
Multi-zone marine protected areas: assessment of ecosystem and fisheries benefits using multiple ecosystem models X. Corrales, D. Vilas, C. Piroddi, J. Steenbeek, Joachim Claudet, J. Lloret, A. Calò, A. Di Franco, T. Font, A. Ligas, et al. To cite this version: X. Corrales, D. Vilas, C. Piroddi, J. Steenbeek, Joachim Claudet, et al.. Multi-zone marine pro- tected areas: assessment of ecosystem and fisheries benefits using multiple ecosystem models. Ocean and Coastal Management, Elsevier, 2020, 193, pp.105232. 10.1016/j.ocecoaman.2020.105232. hal- 03034139 HAL Id: hal-03034139 https://hal.archives-ouvertes.fr/hal-03034139 Submitted on 1 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright 1 To be re-submitted to Ocean and Coastal Management 2 Multi-zone marine protected areas: assessment of ecosystem and fisheries 3 benefits using multiple ecosystem models 4 Corrales, X.1,2,3; Vilas, D.1,2,4,5; Piroddi, C.6; Steenbeek, J.2; Claudet, J.7; Lloret, J.8; Calò, 5 A.9,10; Di Franco, A.9,11; Font, T.8; Ligas, A.12; Prato, G.13; Sahyoun, R.13; Sartor, P.12; 6 Guidetti, P.9,14; Coll, M.1,2. -
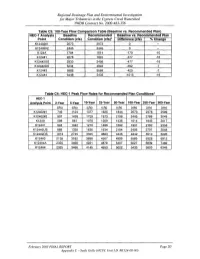
Regional Drainage Plan and Environmental Investigation for Major Tributaries in the Cypress Creek Watershed TWDB Contract No
Regional Drainage Plan and Environmental Investigation for Major Tributaries in the Cypress Creek Watershed TWDB Contract No. 2000-483-356 Table C3: 100-Year Flow Comparison Table (Baseline vs. Recommended Plan) HEC-1 Analysis Baseline Recommended Baseline vs. Recommended Plan Point Condition (cfs) Condition (cfs)· Difference (cfs) "10 Change K12402#1 2073 2073 0 -- K12402#2 2445 2445 0 -- K124A 1784 1614 -170 -10 K124#1 2278 1901 -377 -16 K124#2US 2933 2456 -477 -16 K124#2DS 5234 4842 -392 -7 K124#3 5989 5569 -420 -7 K124#4 6448 5433 -1015 -16 Table C4' HEC-1 Peak Flow Rates for Recommended Plan Conditions· HEC-1 Analysis Point 2-Year S-Year 10-Year 2S-Year SO-Year 100-Year 2S0-Year SOO-Year (cis) (cis) (cis) (cis) (cis) (cis) (cis) ~cls) K12402#1 746 1124 1377 1625 1844 2073 2376 2599 K12402#2 937 1428 1729 1973 2199 2445 2788 3049 K124A 588 881 1076 1269 1436 1614 1845 2017 K124#1 694 1042 1270 1496 1692 1901 2162 2358 K124#2US 889 1339 1636 1934 2184 2456 2797 3048 K124#2DS 1813 2745 3355 3883 4346 4842 5510 6026 K124#3 2136 3182 3898 4507 4999 5569 6328 6912 K124#4A 2325 3466 4221 4878 5407 6027 6839 7462 K124#4 2325 3466 4145 4653 5022 5433 5953 6349 February 2003 FINAL REPORT Page 20 Appendix C - Seals Gully (HCFC Unit I.D. #KI24-00-00) Regional Drainage Plan and Environmental Investigation for Major Tributaries in the Cypress Creek Watershed TWDB Contract No. 2000-483-356 Table C5: Comparison of Water Surface Elevations (100-Year) Seals Gully (K124-00-00' Baseline Condition Recommended Plan Difference Station Location Flow WSEL -

Marine Protection in America's Ocean
SeaStates 2021 Marine Protection in America’s Ocean MARINE PROTECTION ATLAS SeaStates 2021 Marine Protection in America’s Ocean SeaStates 2021 Marine Protection in America’s Ocean SeaStates 2021 is a rigorous, quantitative accounting of fully and words marine reserve or marine protected area are used. Protect- strongly protected MPAs in America’s ocean updated annually by ing biodiversity in marine reserves increases the abundance and Sardines swirl amongst giant kelp off Anacapa Island, part the team at MPAtlas.org. First published in 2013, our annual reports diversity of marine life inside the reserve and typically results in of the Channel Islands National Marine Sanctuary. Federal are intended to measure US progress as a whole, and state prog- the export of marine life to surrounding areas4,5. In short, no-take and state authorities co-manage the MPA complex in the Channel Islands which includes a network of fully protected ress towards effective marine protection in their coastal waters. MPAs effectively protect marine life, can secure food resources areas surrounded by the highly protected sanctuary zone. Oceans are essential to human survival and prosperity, yet our for millions of people by exporting fish outside the MPA, and Marine Conservation Institute has recognized the Northern Channel Islands with a platinum-level Blue Parks award. activities are damaging many ecosystems and pushing numerous prevent loss of species. Strongly protected areas are those in (credit Robert Schwemmer, NOAA) critical marine species toward extinction1. Many marine biologists which extraction is quite limited and conservation benefits are high but not as high as fully protected areas. -

Guidelines for Marine Protected Areas
Guidelines for Marine Protected Areas World Commission on Protected Areas (WCPA) Guidelines for Marine MPAs are needed in all parts of the world – but it is vital to get the support Protected Areas of local communities Edited and coordinated by Graeme Kelleher Adrian Phillips, Series Editor IUCN Protected Areas Programme IUCN Publications Services Unit Rue Mauverney 28 219c Huntingdon Road CH-1196 Gland, Switzerland Cambridge, CB3 0DL, UK Tel: + 41 22 999 00 01 Tel: + 44 1223 277894 Fax: + 41 22 999 00 15 Fax: + 44 1223 277175 E-mail: [email protected] E-mail: [email protected] Best Practice Protected Area Guidelines Series No. 3 IUCN The World Conservation Union The World Conservation Union CZM-Centre These Guidelines are designed to be used in association with other publications which cover relevant subjects in greater detail. In particular, users are encouraged to refer to the following: Case studies of MPAs and their Volume 8, No 2 of PARKS magazine (1998) contributions to fisheries Existing MPAs and priorities for A Global Representative System of Marine establishment and management Protected Areas, edited by Graeme Kelleher, Chris Bleakley and Sue Wells. Great Barrier Reef Marine Park Authority, The World Bank, and IUCN. 4 vols. 1995 Planning and managing MPAs Marine and Coastal Protected Areas: A Guide for Planners and Managers, edited by R.V. Salm and J.R. Clark. IUCN, 1984. Integrated ecosystem management The Contributions of Science to Integrated Coastal Management. GESAMP, 1996 Systems design of protected areas National System Planning for Protected Areas, by Adrian G. Davey. Best Practice Protected Area Guidelines Series No. -

Technical Supplement 14P--Gullies and Their Control
Technical Gullies and Their Control Supplement 14P (210–VI–NEH, August 2007) Technical Supplement 14P Gullies and Their Control Part 654 National Engineering Handbook Issued August 2007 Cover photo: Gully erosion may be a significant source of sediment to the stream. Gullies may also form in the streambanks due to uncontrolled flows from the flood plain (valley trenches). Advisory Note Techniques and approaches contained in this handbook are not all-inclusive, nor universally applicable. Designing stream restorations requires appropriate training and experience, especially to identify conditions where various approaches, tools, and techniques are most applicable, as well as their limitations for design. Note also that prod- uct names are included only to show type and availability and do not constitute endorsement for their specific use. (210–VI–NEH, August 2007) Technical Gullies and Their Control Supplement 14P Contents Purpose TS14P–1 Introduction TS14P–1 Classical gullies and ephemeral gullies TS14P–3 Gullying processes in streams TS14P–3 Issues contributing to gully formation or enlargement TS14P–5 Land use practices .........................................................................................TS14P–5 Soil properties ................................................................................................TS14P–6 Climate ............................................................................................................TS14P–6 Hydrologic and hydraulic controls ..............................................................TS14P–6 -

CHAPTER 5 Ecopath with Ecosim: Linking Fisheries and Ecology
CHAPTER 5 Ecopath with Ecosim: linking fi sheries and ecology V. Christensen Fisheries Centre, University of British Columbia, Canada. 1 Why ecosystem modeling in fi sheries? Fifty years ago, fi sheries science emerged as a quantitative discipline with the publication of Ray Beverton and Sidney Holt’s [1] seminal volume On the Dynamics of Exploited Fish Populations. This book provided the foundation for how to manage fi sheries and was based on detailed, mathe- matical analyses of the dynamics of individual fi sh populations, of how they grow and how they are affected by fi shing. Fisheries science has developed and matured since then, and remarkably much of what has been achieved are modifi cations and further developments of what Beverton and Holt introduced. Given then that fi sheries science has developed to become one of the most data-rich, quantita- tive fi elds in ecology [2], how well has it fared? We often see fi sheries issues in the headlines and usually in a negative context and there are indeed many threats to the sustainability of ocean resources [3]. Many, judging not the least from newspaper headlines, consider fi sheries manage- ment a usual suspect in connection with fi sheries collapses. This may lead one to suspect that there is a problem with the science, but I hold this to be an erroneous conclusion. It should be stressed that the main problem is not to be found in the computational aspects of the science, but rather in how management advice actually is implemented in praxis [4]. The major force in fi sh- eries throughout the world is excessive fi shing capacity; the days with unexploited resources and untapped oceans are over [5], and the fi shing industry is now relying heavily on subsidies to keep the machinery going [6].